Your Location:Home >Products >OLED intermediates >Thiophenes >139100-06-4


Product Details
Chemical Properties
Colorless to yellow liquid
Uses
2-Bromo-3-dodecylthiophene is used in the synthesis of a poly(octathiophene) based copolymer, which is an organic semiconductor that may be used in thin film transistor.
InChI:InChI=1/C16H27BrS/c1-2-3-4-5-6-7-8-9-10-11-12-15-13-14-18-16(15)17/h13-14H,2-12H2,1H3
Three copolymers of benzo[1,2-b:4,5-b']dithiophene and 3,3'-bis(alkyl)-5,5'-bithiophene (dodecyl, tetradecyl and hexadecyl side chains) have been synthesized through Stille copolymerization. The polymers have number-average molecular weights over 20 kg/mol, are well-packed in the bulk and thin film, and possess an ionization potential of -5.1 eV in thin film, which offers stability versus oxidation in environmental conditions. The thin film packing of the polymer with dodecyl side chains leads to an excimeric emission upon excitation, which is not observed for longer side chain lengths. The presence of the dimers responsible for this excimer formation results in a device performance improvement as well. Field-effect transistors fabricated from these copolymers have On/Off ratios >107, equal saturation and linear hole mobilities above 10-2 cm2/Vs, almost no hysteresis and turn-on voltages around 0 V in bottom-contact devices.
Fine tuning the energy levels of donor polymers is a critically important step toward achieving high power conversion efficiencies in polymer solar cells (PSCs). We systematically controlled the energy levels of donor polymers by introducing cyano (CN) and alkoxy (OR) groups into the 4,4′-didodecyl-2,2′-bithiophene (BT) unit in a step-by-step fashion, thereby varying the inductive and resonance effects. The three monomer units (BT, BTC, and BTCox) were polymerized with benzo[1,2-b:4,5-b′]dithiophene (BDT) as a counter unit to afford three polymers (PBDT-BT, PBDT-BTC, and PBDT-BTCox). The highest occupied molecular orbital and lowest unoccupied molecular orbital energy levels decreased significantly upon the introduction of CN groups, and these levels increased slightly upon attachment of the OR groups, in good agreement with the measured open-circuit voltages of the three polymer devices. The strong inductive and resonance effects present in PBDT-BTCox narrowed the polymer band gap to 1.74 eV to afford a power conversion efficiency of 5.06%, the highest value achieved among the three polymers.
We present here the synthesis, characterization, and field-effect performance of a novel n-channel semiconducting molecule TIFDMT and of the corresponding thiophene-based copolymer P-IFDMT4 based on the indenofluorenebis(dicyanovinylene) core. TIFDMT-based field-effect transistors fabricated by spin-coating exhibit high electron mobilities of 0.10-0.16 cm2/V s in air, low turn-on voltages (~0 to +5 V), and high on/off ratios of 107-108. These devices also exhibit excellent air stability over a prolonged time of storage in ambient conditions. P-IFDMT4-based devices exhibit the first example of an air-stable ambipolar polymer processable from solution. Copyright
New push-pull copolymers based on thiophene (donor) and benzothiadiazole (acceptor) units, poly[4,7-bis(3-dodecylthiophene-2-yl) benzothiadiazole-co- thiophene] (PT3B1) and poly[4,7-bis(3-dodecylthiophene-2-yl) benzothiadiazole-co-benzothiadiazole] (PT2B2), are designed and synthesized via Stille and Suzuki coupling routes respectively. Gel permeation chromatography shows the number average molecular weights are 31100 and 8400 g mol-1 for the two polymers, respectively. Both polymers have shown absorption throughout a wide range of the UV-vis region, from 300 to 650 nm. A significant red shift of the absorption edge is observed in thin films compared to solution of the copolymers; the optical band gap is in the range of 1.7 to 1.8 eV. Cyclic voltammetry indicates reversible oxidation and reduction processes with HOMO energy levels calculated to be in the range of 5.2 to 5.4 eV. Upon testing both materials for organic field-effect transistors (OFETs), PT3B1 showed a hole mobility of 6.1 × 10-4 cm2 V-1 s -1, while PT2B2 did not show any field effect transport. Both copolymers displayed a photovoltaic response when combined with a methanofullerene as an electron acceptor. The best performance was achieved when the copolymer PT3B1 was blended with [70]PCBM in a 1:4 ratio, exhibiting a short-circuit current of 7.27 mA cm-2, an open circuit voltage of 0.85 V, and a fill factor of 41% yielding a power conversion efficiency of 2.54% under simulated air mass (AM) 1.5 global (1.5 G) illumination conditions (100 mW cm-2). Similar devices utilizing PT2B2 in place of PT3B1 demonstrated reduced performance with a short-circuit current of 4.8 mA cm -2, an open circuit voltage of 0.73 V, and a fill factor of 30% resulting in a power conversion efficiency of roughly 1.06%.
Bolaamphiphilies with D-A-D type π-conjugated rigid cores composed by thiophenes as donors (D) and benzothiadiazole (BTD) as central acceptor (A) have been synthesised. Their self-assemblies and photophysical properties were investigated by polarising optical microscope, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. Such compounds can self-assemble into honeycomb cylinder mesophases with Colhex?/p6mm and Colsqu/p4mm lattices in their pure states as well as organogels with different morphologies in organic solvents. Their absorption spectra cover nearly the entire visible light range and their band gaps are relatively low. Tetrathiophene BTD based bolaamphilphiles (BT4/n) with higher D/A ratios than the bisthiophene BTD bolaamphilphiles (BT2/n) can self-assemble into more ordered nanostructures in both bulk states and solution. Both the absorption and emission peaks of BT4/n are strongly red shifted. The influence of the molecular conformation, the conjugated core length, as well as the D/A ratio on the self-assemble and photophysical characteristics of such D-A-D bolaamphiphiles are discussed.
In this study, we obtained a new structural insight into the charge-transporting properties in TPD-based polymers that cannot be solely explained in terms of the type of orientation. We synthesized two types of copolymers comprising mono-TPD or bis-TPD as the accepting unit. Although the planarity and energy levels are similar with the mono-TPD unit, the aggregation state is quite different, and the X-aggregation tendency seems to be stronger when the bis-TPD unit is incorporated. In the case of TPD1, an effective π-πorbital overlap is found to originate from the H-aggregates, and 3D charge transport pathways are formed with a bimodal orientation of edge-on and face-on, resulting in an efficient charge transportation (1.84 cm2·V-1·s-1 of hole and 0.31 cm2·V-1·s-1 of electron). In contrast, despite the well-aligned edge-on orientation of TPD2, it exhibited a relatively very low mobility and splitted emission characteristics in photoluminescence spectra because of the tilted intermolecular stacking pattern with an X-shape (0.015 cm2·V-1·s-1 for hole and 0.16 cm2·V-1·s-1 for electron). An overall characterization of the semiconducting polymers was performed, and it was found that the type of aggregation in the final thin films, such as H- or X-aggregation, is indeed important and perhaps more important than the orientation to obtain polymers with a high charge carrier mobility.
Precise control of the molecular arrangements at the interface between the electron donor and acceptor in mixed bulk heterojunctions (BHJs) remains challenging, despite the correlation between structural characteristics and efficiency in organic photovoltaics (OPVs). This study reveals that the substitution patterns of linear and branched alkyl side chains on electron-donating/-accepting alternating copolymers can control the positions of an acceptor molecule (C60) around the π-conjugated main chains in mixed BHJs. Two-dimensional solid-state NMR demonstrates a marked difference in the location of C60 in the blend films. A copolymer with an electron-accepting unit positioned in close proximity to C60 demonstrated higher OPV performance in combination with various fullerene derivatives. This molecular design offers precise control over the interfacial molecular structure, thereby paving the way for overcoming the current limitations of OPVs comprising mixed BHJs.
The invention aims at designing and synthesizing a cyanogroup compound and modifying a perovskite layer in a perovskite solar cell structure. According to an implementation method, a layer of modification material is arranged on the surface of perovskite in a spin coating manner, cyanogroups in material molecules and I in the perovskite structure interact, surface charges of the modification material are dispersed, meanwhile, I migration is reduced, and therefore the stability of the perovskite layer is improved; and benzene ring and alkyl chain components in the molecular structure can achieve the functions of improving interfacial compatibility and reducing the surface defects, and finally the aim of improving the performance of a perovskite solar cell is achieved. Ar in a chemical formula (1) (please see the specification) is a following aromatic compound (please see the specification). Ar in a chemical formula (2) (please see the specification) is a following aromatic compound (please see the specification). R1 is -CN or a chemical formula (please see the specification), and R2 is 1-16 alkyl chains.

3-dodecylthiophene


2-bromo-3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
|
97% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 24h;
|
94% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
for 1.75h;
|
93% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
Inert atmosphere;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 - 20 ℃;
|
93% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
|
93% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
for 6h;
Inert atmosphere;
Schlenk technique;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform;
at 20 ℃;
|
92% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
|
90% |
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
Darkness;
|
86% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 2h;
|
82% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 40h;
|
75% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 ℃;
for 2h;
Inert atmosphere;
Darkness;
|
74% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
41% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform; N,N-dimethyl-formamide;
at 0 ℃;
for 1h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
Inert atmosphere;
|
1.36 g |
|
With
N-Bromosuccinimide; N,N-dimethyl-formamide;
In
chloroform;
at 40 ℃;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
|
laurylmagnesium bromide


2-bromo-3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 73 percent / Ni(dppp)Cl2 / diethyl ether / 35 °C
2: 41 percent / Br2 / acetic acid / 0.5 h / 0 °C
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); bromine;
In
diethyl ether; acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 20 - 60 °C / Inert atmosphere
2: N-Bromosuccinimide / chloroform; acetic acid / 2 h / 20 °C / Inert atmosphere; Darkness
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
diethyl ether; chloroform; acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 15 h / 40 °C
2: N-Bromosuccinimide; acetic acid / chloroform; N,N-dimethyl-formamide / 1 h / 0 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); acetic acid;
In
diethyl ether; chloroform; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 25 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 50 °C
2: N-Bromosuccinimide; acetic acid / chloroform / 20 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); acetic acid;
In
diethyl ether; chloroform;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 40 °C
2: N-Bromosuccinimide; N,N-dimethyl-formamide / chloroform / 40 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N,N-dimethyl-formamide;
In
diethyl ether; chloroform;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 20 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
|

3-dodecylthiophene
laurylmagnesium bromide

1-dodecylbromide

3,3?-didodecyl-2,2′;5′,2″;5″,2?-quaterthiophene
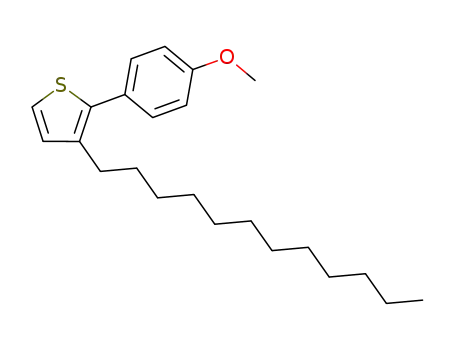
C23H34OS
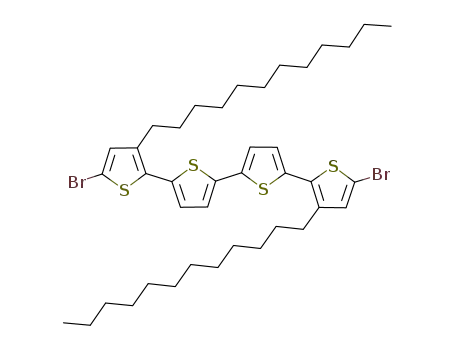
5,5'''-dibromo-3,3'''-bis(dodecyl)-2,2':5',2'':5'',2'''-quarterthiophene
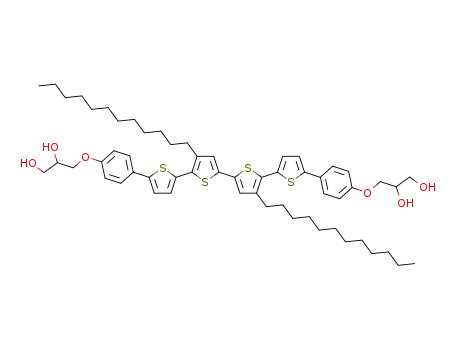
C58H78O6S4
CAS:65344-26-5
CAS:65016-55-9
CAS:104934-52-3