Your Location:Home >Products >OLED intermediates >Thiophenes >3172-56-3
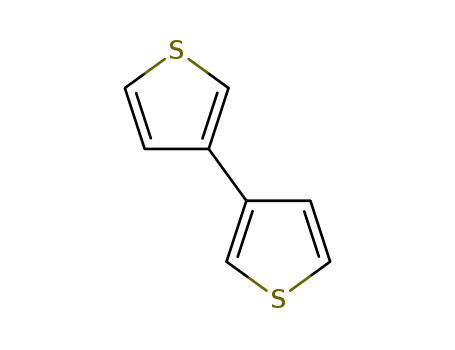

Product Details
Chemical Properties
brown powder
-
Heteroarene-substituted vinyl- and allyl-silanes were obtained in good yields by the cross-coupling reaction of either heteroaryl Grignard reagents with halovinyl- and haloallyl-silanes or, alternatively, silyl- and silylmethyl-substituted vinylmetallic reagents with heteroaryl halides in the presence of PdCl2(dppb) as a catalyst.
An efficient method for the synthesis of 6-thienyl-substituted 2-amino-3-cyanopyridines by the ring transformation in the corresponding pyrimidines was developed. Further modification of the pyridines obtained under conditions of a room temperature aerobic Suzuki reaction in the presence of trans-bis(dicyclohexylamine)palladium(II) acetate as a catalyst was studied
The Fujiwara-Moritani reaction has had a profound contribution in the emergence of contemporary C-H activation protocols. Despite the applicability of the traditional approach in different fields, the associated reactivity and regioselectivity issues had
The construction of three-dimensional (3D) covalent organic frameworks (COFs), especially fully conjugated 3D COFs, is a long-standing challenge. Herein, we report a saddle-like, π-conjugated cyclooctatetrathiophene (COTh) as a tetrahedral node to construct fully conjugated 3D COFs. The present work enriches the structural diversities and potential applications of 3D COFs.
Typically, Suzuki couplings used in polymerizations are performed at raised temperatures in inert atmospheres. As a result, the synthesis of aromatic materials that utilize this chemistry often demands expensive and specialized equipment on an industrial scale. Herein, we describe a bimetallic methodology that exploits the distinct reactivities of palladium and copper to perform high yielding aryl-aryl dimerizations and polymerizations that can be performed on a benchtop under ambient conditions. These couplings are facile and can be performed by simple mixing in the open vessel. To demonstrate the utility of this method in the context of polymer synthesis: polyfluorene, polycarbazole, polysilafluorene, and poly(6,12-dihydro-dithienoindacenodithiophene) were created at ambient temperature and open to air.
The photoelectric properties and catalytic activities of substituted triphenylphosphine and sulfur/selenium ligand supported aromatic triangular tri-palladium complexes1-4, abbreviated as [Pd3]+, were investigated. The cyclic voltammogram of [Pd3]+in CH3CN-nBu4NPF6showed a single quasi-reversible wave which was consistent with their robust property and provided preliminary proof for their electron transfer processes in catalysis. With excitation at 267 nm, [Pd3]+exhibited strong ratiometric fluorescence at 550 and 780 nm at a temperature gradient from 77 K to 287 K. These peculiar triangular tri-palladium complexes showed excellent catalytic activities and exclusive reactivity with aryl iodides over the other halogenated aromatics in the Suzuki-Miyaura coupling reaction. The electronic and steric hindrance effects of substituents on the aryl iodides and aryl boronic acids including heteroaromatics like pyridine, pyrazine and thiophenes were explored and most substrates achieved up to 99% of yields. (2-[1,1′-Biphenyl]-2-ylbenzothiazole) which was analogous to the selective cyclooxygenase-2 (COX-2) inhibitors was also synthesized with our tri-palladium catalyst and gave good isolated yield (94%). The study of the catalytic process revealed that the mechanism of the reaction may involve the replacement of the sulphur ligand on [Pd3]+by iodine from aryl iodides, which was beneficial for the matching of C-I bond energy.
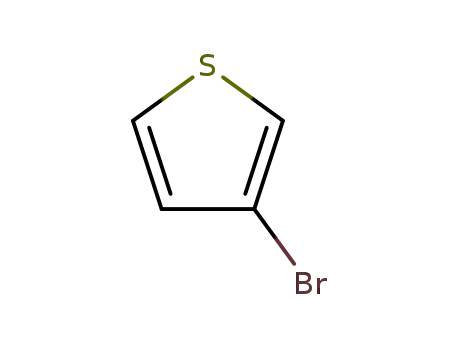
3-Bromothiophene

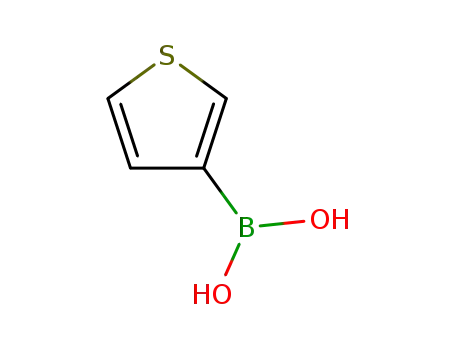
Thien-3-ylboronic acid

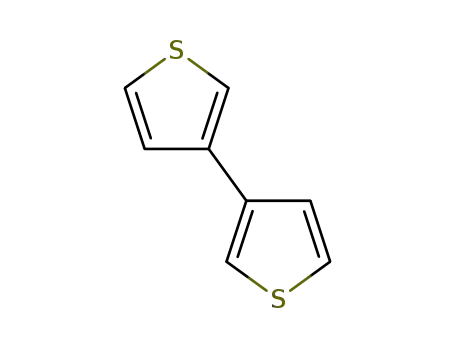
3,3'-bithiophene
| Conditions | Yield |
|---|---|
|
With
bis(dicyclopentyl(2-methoxyphenyl)phosphine)dichloropalladium(II);
In
butan-1-ol;
at 100 ℃;
for 4h;
Cooling;
|
98% |
|
With
tetrakis(triphenylphosphine) palladium(0); sodium carbonate;
In
ethanol; water; toluene;
for 20h;
Inert atmosphere;
Reflux;
|
87% |
|
With
potassium hydroxide;
palladium diacetate; triphenylphosphine;
In
1,2-dimethoxyethane; water;
at 80 ℃;
for 9.25h;
|
76.9% |
|
With
sodium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
1,2-dimethoxyethane; water;
for 10h;
Heating / reflux;
|
55% |
|
With
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran; water;
|

3-Bromothiophene


3,3'-bithiophene
| Conditions | Yield |
|---|---|
|
With
(2-hydroxyethyl)ammonium formate; palladium dichloride;
at 100 ℃;
for 2h;
|
96% |
|
With
PEG 4000; palladium diacetate; potassium carbonate;
at 120 ℃;
for 7h;
|
95% |
|
With
triethylamine;
palladium dichloride;
at 80 ℃;
for 5h;
|
94% |
|
With
2Pd(2+)*4Br(1-)*C56H102N4; potassium carbonate;
at 75 ℃;
for 12h;
|
93% |
|
With
[(PhNH)P2(NPh)2]2NPh; triethylamine; palladium dichloride;
In
water;
for 2h;
Reflux;
|
91% |
|
With
palladium diacetate; sodium hydroxide; agarose;
In
water;
at 90 ℃;
for 2h;
|
91% |
|
With
triethylamine; palladium dichloride;
at 20 - 100 ℃;
for 1.45h;
Green chemistry;
|
90% |
|
With
DPEPhos; potassium tert-butylate; palladium diacetate; bis(pinacol)diborane;
for 12h;
Heating;
|
89% |
|
With
[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride; tert.-butyl lithium;
In
n-heptane;
at 20 ℃;
for 0.166667h;
Schlenk technique;
|
89% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
With
copper dichloride;
In
diethyl ether;
Further stages.;
|
87% |
|
With
tetrabutylammonium acetate; palladium diacetate; propionaldehyde;
at 90 ℃;
for 1h;
Inert atmosphere;
|
86% |
|
With
D-glucose; tetra(n-butyl)ammonium hydroxide; palladium diacetate;
In
water;
at 40 ℃;
for 6h;
|
86% |
|
3-Bromothiophene;
With
tert.-butyl lithium;
In
diethyl ether;
at -78 ℃;
In
diethyl ether;
With
Duroquinone;
In
diethyl ether;
|
82% |
|
With
iron(III) trifluoromethanesulfonate; magnesium;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
|
81% |
|
With
manganese; zirconocene dichloride; [(2,9-dimethyl-1,10-phenanthroline)dichloro nickel(II)]; lithium chloride;
In
1,2-dimethoxyethane;
at 20 ℃;
for 48h;
Inert atmosphere;
|
80% |
|
With
potassium phosphate; triphenylphosphine; bis(pinacol)diborane;
cyclopalladated ferrocenylimine;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 14h;
|
78% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Inert atmosphere;
|
77.9% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 - -60 ℃;
for 1.16667h;
Schlenk technique;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether; hexane;
at -60 - 20 ℃;
for 19h;
Schlenk technique;
Inert atmosphere;
|
76% |
|
With
n-butyllithium; copper dichloride;
In
diethyl ether;
at -78 - 20 ℃;
Inert atmosphere;
|
73.9% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide; samarium; nickel dichloride;
In
tetrahydrofuran;
for 24h;
chemoselective reaction;
Inert atmosphere;
Reflux;
|
73% |
|
With
potassium carbonate;
In
ethanol; water;
at 20 ℃;
for 12h;
|
73% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -70 ℃;
for 0.25h;
With
copper dichloride;
In
diethyl ether; hexane;
at -45 ℃;
for 1h;
|
71% |
|
With
tetrabutylammomium bromide; potassium carbonate; hydroquinone;
dichloro bis(acetonitrile) palladium(II); N,N'-dicyclohexylethylenediimine;
In
N,N-dimethyl-formamide;
at 135 ℃;
for 24h;
|
71% |
|
With
palladium diacetate; bis(tri-n-butyltin); cesium fluoride; tricyclohexylphosphine;
In
neat (no solvent);
at 110 ℃;
for 24h;
|
69% |
|
With
thio-xanthene-9-one; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine]nickel(II) dichloride; N-ethyl-N,N-diisopropylamine;
In
tert-butyl alcohol;
at 20 ℃;
for 12h;
Schlenk technique;
Irradiation;
|
68% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether; hexane;
at -70 - -55 ℃;
With
copper dichloride;
In
diethyl ether; hexane;
at -45 ℃;
for 1h;
|
65% |
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
Inert atmosphere;
With
copper(l) chloride;
In
diethyl ether;
for 3h;
Inert atmosphere;
Reflux;
|
64% |
|
With
[2,2]bipyridinyl; nickel diacetate; sodium tert-pentoxide;
In
tetrahydrofuran;
at 25 ℃;
for 0.000833333h;
|
60% |
|
3-Bromothiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
With
copper dichloride;
|
60% |
|
With
bis(bipyridine)nickel(II) bromide; ethylene dibromide; sodium iodide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
Electrochemical reaction;
Inert atmosphere;
|
48% |
|
With
manganese; tetrakis(triphenylphosphine) palladium(0); ethylene dibromide;
Yield given. Multistep reaction;
1.) THF, 5 h, room t., 2.) THF, 0 deg C to room t., 20 min, 3.) THF, room t., 30 min;
|
|
|
With
pyridine; tetrabutylammonium tetrafluoroborate;
Ethyl 4-bromobenzoate;
In
acetonitrile;
at 20 ℃;
Electrolysis;
iron rod as anode; stainless steel grid as cathode;
|
|
|
3-Bromothiophene;
With
TurboGrignard;
With
synthetic air; cobalt(II) chloride;
|
|
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
for 0.25h;
Inert atmosphere;
With
copper dichloride;
In
diethyl ether;
at -55 - 20 ℃;
|
|
|
3-Bromothiophene;
With
n-butyllithium;
In
diethyl ether;
at -78 ℃;
With
copper dichloride;
In
diethyl ether;
at -78 ℃;
|
thiophene
thiophene
3-chlorothiophene

3-Bromothiophene
2,2′-dibromo-[3,3′]bithiophene
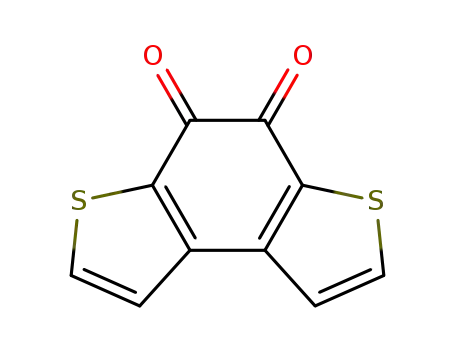
benzo[1,2-b:4,3-b’]dithiophene-4,5-quinone
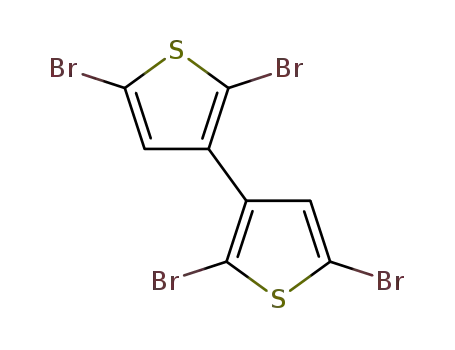
2,2’,5,5’-tetrabromo-3,3’-dithienyl
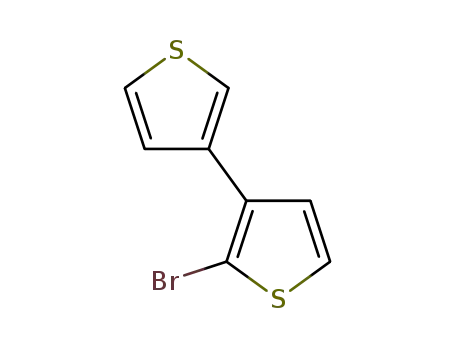
2-bromo-3,3'-dithiophene
CAS:1081-34-1
CAS:116971-10-9