Your Location:Home >Products >OLED intermediates >Thiophenes >3141-24-0
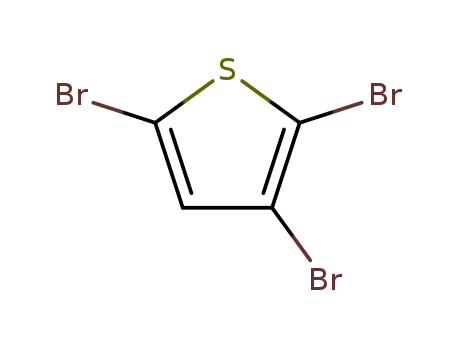

Product Details
Chemical Properties
white to light yellow crystal powder
Uses
2,3,5-Tribromothiophene was used in the synthesis of 2,5-bis(dicyanomethylene)-3-bromo-2,5-dihydrothiophene.
InChI:InChI=1/C4HBr3S/c5-2-1-3(6)8-4(2)7/h1H
2,2′,5,5′-Tetraphenyl-3,3′-bithiophene-4,4′-diol, the first member of a new class of chiral C2-symmetric atropisomeric diols based on a biheteroaromatic scaffold, has been synthesized. Dynamic enantioselective HPLC experiments revealed a racemization barrier of 22.9 kcal/mol at 25 °C, which is high enough to permit the chromatographic collection of both enantiomers on a semi-preparative scale, but at the borders of application as a promoter in asymmetric catalysis. The ground and transition states involved in the enantiomerization process have been identified through DFT calculations, which suggest the intermediacy of a tautomeric species in which a thiophen-3-ol unit and a 2,3-dihydrothiophen-3-one ring are present. The proposed racemization mechanism is supported by the results of dynamic HPLC experiments. Calculations also suggested which substituent groups at the 2,2′-positions of the thiophene rings could lead to an increase in enantiomerization barriers sufficient to confer full configurational stability on the 3,3′-bithiophene framework.
Dinuclear carbene complexes with 3,6-dimethylthieno[3,2-b] thienylene (T′T′) and thieno[3,2-b]thienylene (TT) spacers were prepared using the classical Fischer method. Their reactivity in solution towards oxygen, as well as their structural features were investigated. Chromium and tungsten carbene complexes were synthesized via dilithiation of the thienothiophene, followed by the addition of the appropriate metal carbonyl complex and subsequent alkylation with triethyloxonium tetrafluoroborate. Monocarbene [M{C(OEt)T′T′H}-(CO)5], (M = Cr, 1; W, 4), biscarbene [(CO)5M{C-(OEt) T′T′C(OEt)}M(CO)5], (M = Cr, 2; W, 5), and the products [M{C(OEt)T′T′C(O)OEt} (CO)5], (M = Cr, 3; W, 6), where a metal moiety of 2 and 5 has been replaced by an ester functionality, were isolated. The reaction product afforded in the reaction of Cr(CO)6 with lithiated thieno[3,2-b]thiophene, [Cr{C(OEt)TTC(O)Bu}(CO)5], 7, did not resemble any of the products isolated in the similar reaction of Cr(CO)6 with lithiated 3,6-dimethylthieno [3,2-b]thiophene.
A photochromic diarylethene having methoxymethyl groups at the reactive carbons was synthesized, and the thermal stability of the closed-ring isomer was examined. The closed-ring isomer of the diarylethene caused both the thermal cycloreversion and a thermally irreversible reaction at 120°C. The structure of the thermal product was determined by 1H NMR, MS, and X-ray crystallographic analysis.
The invention relates to an air catalytic oxidation hydrogen bromide method for preparing 2,3,5-tribromo thiophene. The air catalytic oxidation hydrogen bromide method is characterized by including the following specific steps: chloroform or petroleum ether, thiophene and cupric bromide or sodium nitride or iron bromide or cobalt bromide or ruthenium bromide are put into a reaction kettle (1) to be stirred, wherein the volume ratio of the chloroform or the petroleum ether to the thiophene is 2 to 1, and the cupric bromide or the sodium nitride or the iron bromide or the cobalt bromide or the ruthenium bromide comprises, by mass, 1% of thiophene; then bromine is dropwise added into the reaction kettle (1) through a first dropwise-adding tank (6) for reacting, and cooling is carried out; then an 0.05-mol/L-sodium-hydroxide ethanol solution with the volume a half of the volume of the bromine is dropwise added into the reaction kettle (1) through a second dropwise-adding tank (7); the reaction mixture is placed into a water washing kettle (2), water with the volume the same as the volume of the chloroform or the petroleum ether is added, sufficient stirring and standing are carried out, the mixture in the organic phase is charged into a distillation kettle (3) for atmospheric distillation, solvents and front cut fractions are recycled into a solvent recycling tank (4), then reduced pressure distillation is carried out, and 112-DEG C-116-DEG C/15 mmHg cut fractions are collected into a product collecting tank (5) to obtain the colorless liquid 2,3,5-tribromo thiophene. The air catalytic oxidation hydrogen bromide method is low in production cost and small in environment pollution, and the bromine use rate is higher than 85%.
We describe the structural and variable temperature magnetic susceptibility properties of an unusual homoleptic bimetallic iron(iii) thiocyanate tetraanion. This work represents the first structurally characterized bis(μ-1,3-thiocyanato) dimer of iron(iii). A weak antiferromagnetic exchange interaction is observed between the two iron(iii) ions, which is supported by broken symmetry density functional theory (DFT) calculations.
The present invention is directed to inhibitors of S-nitrosoglutathione reductase (GSNOR), pharmaceutical compositions comprising such GSNOR inhibitors, and methods of making and using the same.
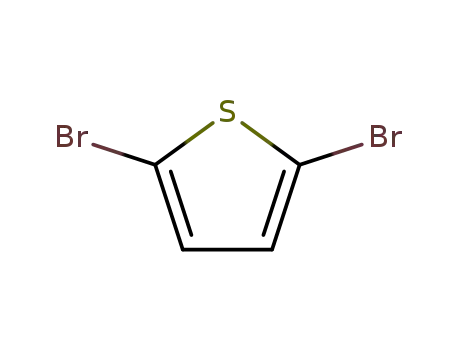
2,5-dibromothiophen

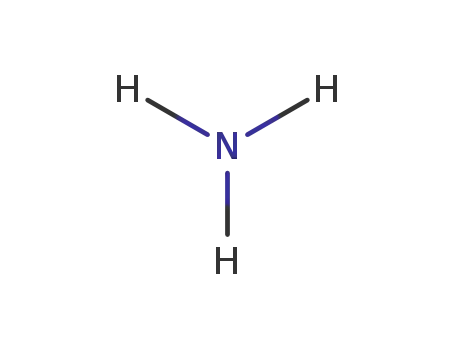
ammonia

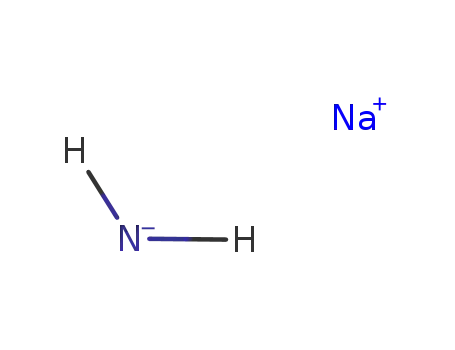
sodium amide

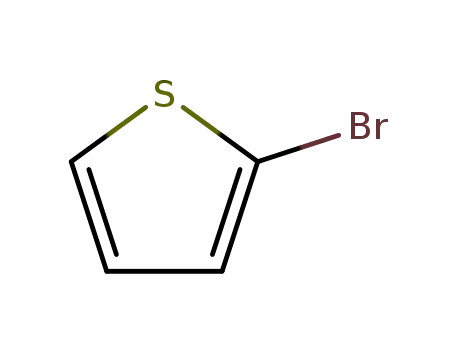
2-bromothiophene

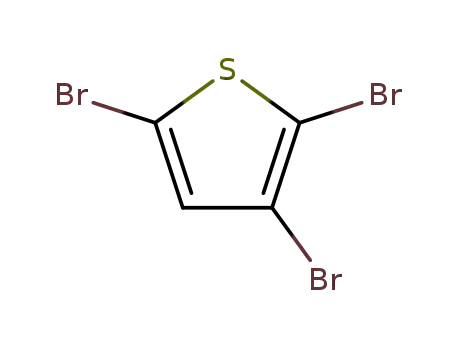
2,3,5-tribromothiophene

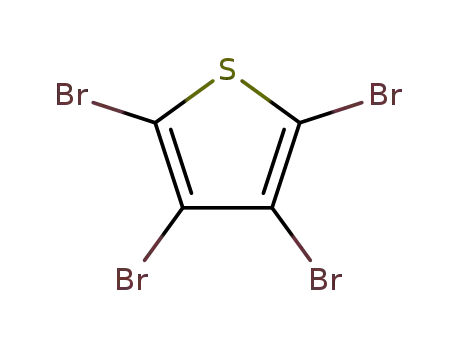
Tetrabromothiophene
| Conditions | Yield |
|---|---|
|
|
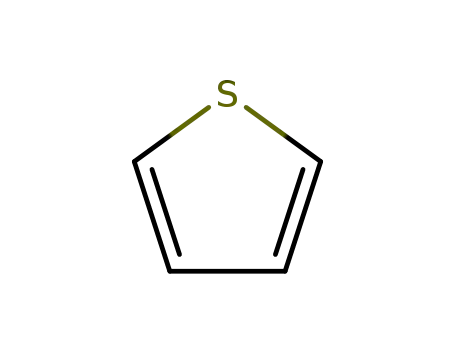
thiophene

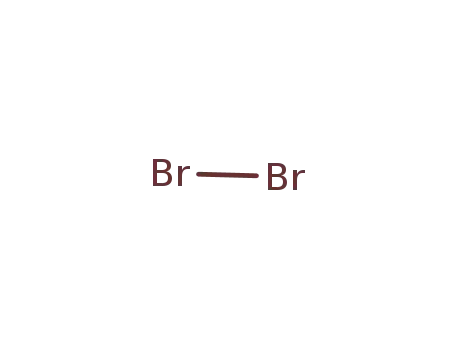
bromine


2-bromothiophene

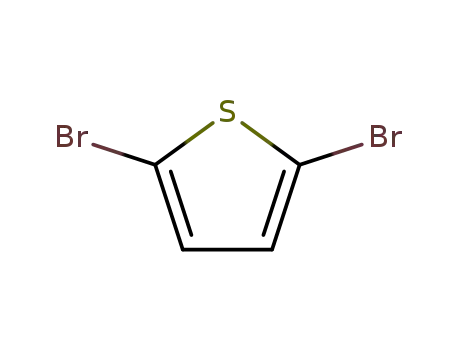
2,5-dibromothiophen


2,3,5-tribromothiophene


Tetrabromothiophene
| Conditions | Yield |
|---|---|
|
|

thiophene
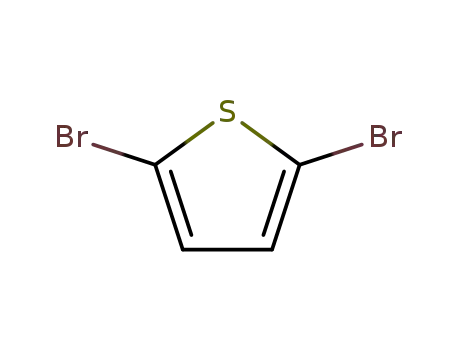
2,5-dibromothiophen

2-bromothiophene

Tetrabromothiophene
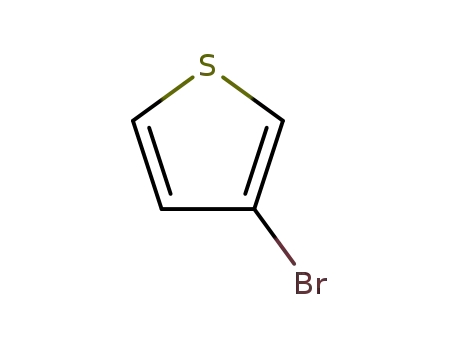
3-Bromothiophene
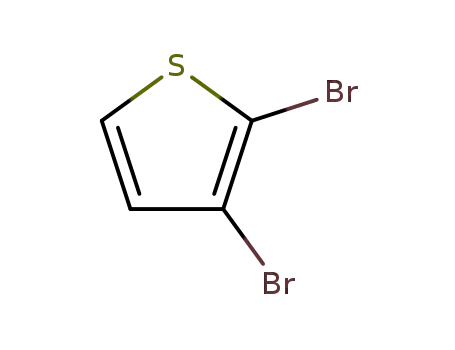
2,3-dibromothiophen
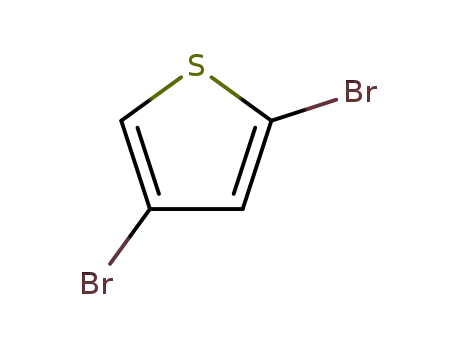
2,4-dibromothiophene
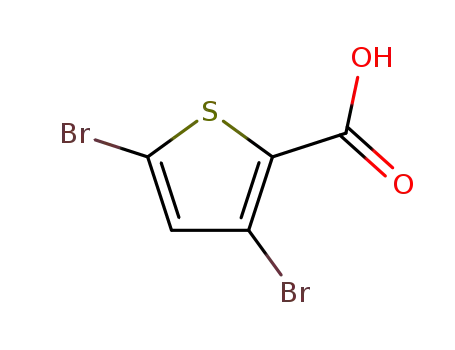
3,5-dibromothiophene-2-carboxylic acid
CAS:1402393-56-9
Molecular Formula:C23H31BrO2
Molecular Weight:419.4
CAS:145965-14-6
CAS:3140-93-0