Your Location:Home >Products >OLED intermediates >Boric acids >145965-14-6
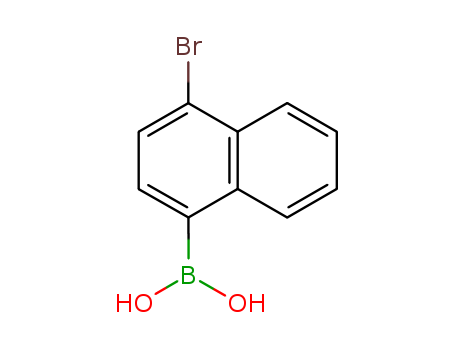

Product Details
In order to systematically explore and understand the structure-activity relationship (SAR) of a lesinurad-based hit (1c) derived from the replacement of the S atom in lesinurad with CH2, 18 compounds (1a-1r) were designed, synthesized and subjected to in vitro URAT1 inhibitory assay. The SAR exploration led to the discovery of a highly potent flexible URAT1 inhibitor, 1q, which was 31-fold more potent than parent lesinurad (IC50 = 0.23 μM against human URAT1 for 1q vs. 7.18 μM for lesinurad). The present study discovered a flexible molecular scaffold, as represented by 1q, which might serve as a promising prototype scaffold for further development of potent URAT1 inhibitors, and also demonstrated that the S atom in lesinurad was not indispensable for its URAT1 inhibitory activity.
The invention relates to thiophene derivatives used as an inhibitor of urate transporter 1 (URAT1), in particular relates to the thiophene derivatives represented by a general formula (I) shown in thedescription, a pharmaceutically-acceptable salt of the thiophene derivatives, and preparation methods of the thiophene derivatives and the pharmaceutically-acceptable salt, and particularly relates to an application of the thiophene derivatives and the pharmaceutically-acceptable salt used as a therapeutic agent for a disease associated with an abnormal uric acid level.
Provided are a diamine compound and an organic light-emitting device including the same. The organic light-emitting device includes: a first electrode; a second electrode; and an organic layer between the first electrode and the second electrode and comprising an emission layer, the organic layer including a diamine compound represented by one selected from Formulae 1-1 to 1-8.
The present invention provide a diamine compound and an organic light-emitting device including the same. The organic light-emitting device includes: a first electrode; a second electrode; and an organic layer between the first electrode and the second electrode and comprising an emission layer, the organic layer including a diamine compound represented by one selected from Formulae 1-1 to 1-8.
The invention provides a series of composite with a formula (I), wherein n is selected from 1, 2 or 3, X is selected from O or S, Q is selected from C or N, and R is selected from H, halogen or -C1-6.The composite can be used as the URAT 1 inhibitor and can be used for treating hyperuricemia and gout.
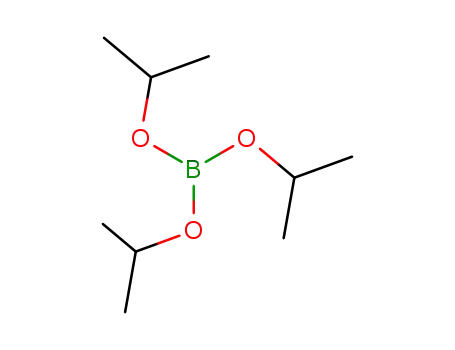
Triisopropyl borate

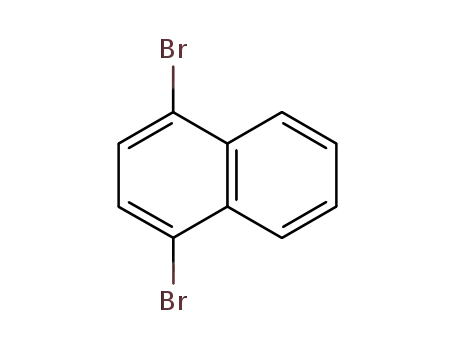
1,4-dibromonaphthalene

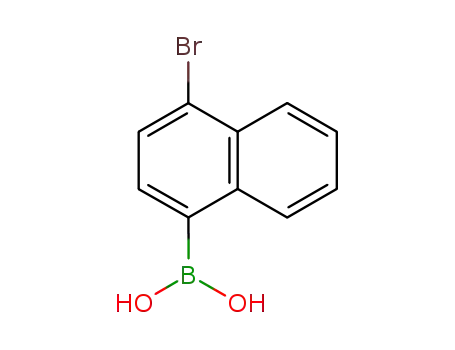
1-bromo-4-(dihydroxyboryl)naphthalene
| Conditions | Yield |
|---|---|
|
1,4-dibromonaphthalene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at 20 ℃;
|
76% |
|
1,4-dibromonaphthalene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 1h;
|
|
|
1,4-dibromonaphthalene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at 20 ℃;
|
1.5 g |
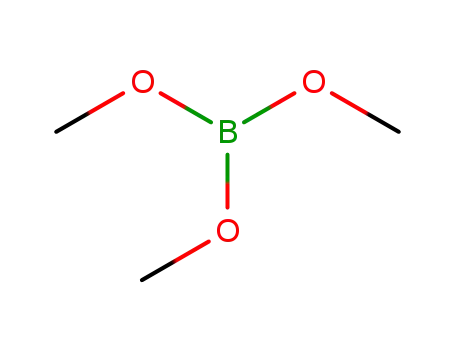
Trimethyl borate


1,4-dibromonaphthalene


1-bromo-4-(dihydroxyboryl)naphthalene
| Conditions | Yield |
|---|---|
|
1,4-dibromonaphthalene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 15h;
With
hydrogenchloride;
In
tetrahydrofuran; hexane; water;
for 1h;
|
82% |
|
1,4-dibromonaphthalene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
|
82% |

Triisopropyl borate

1,4-dibromonaphthalene
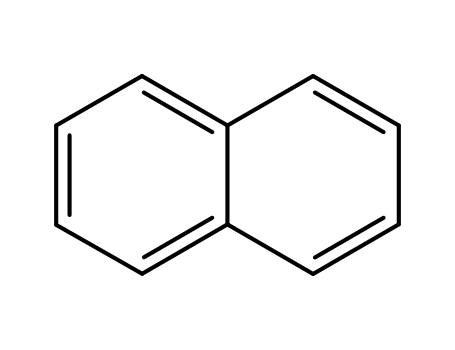
naphthalene

Trimethyl borate
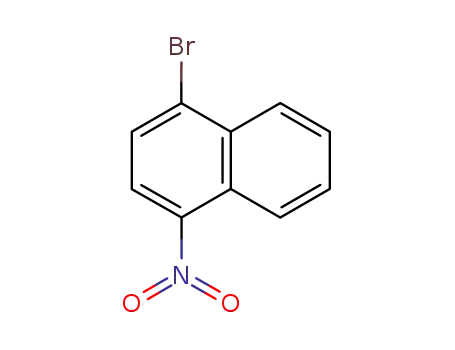
4-bromo-1-nitronaphthalene
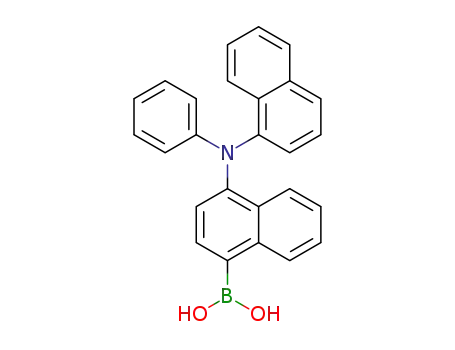
(4-(naphthalen-1-yl(phenyl)amino)naphthalen-1-yl)boronic acid
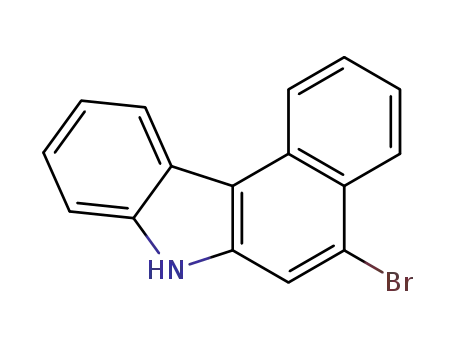
5-bromo-7H-benzo[c]carbazole
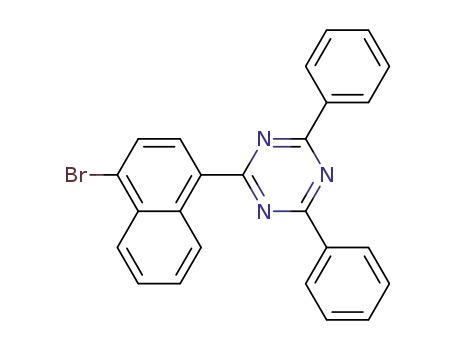
2-(4-bromonaphthalene-1-yl)-4,6-diphenyl-1,3,5-triazine
CAS:479094-62-7
CAS:27813-21-4
CAS:3141-24-0