Your Location:Home >Products >OLED intermediates >Boric acids >5122-94-1

Product Details
|
Chemical Properties |
Solid |
| Description | Contains varying amounts of anhydride |
|
Uses |
suzuki reaction: Suzuki coupling of 2-(4-bromophenyl)thiophene with phenylboronic acid and 4-biphenylboronic acid are used for the preparation of 4-(2-thieny)biphenyl and 4-(2-thienyl)-1,1':4,1''-biphenyl respectively. Coupling of two equivalents of 4-(2-thienyl)phenylboronic acid with 1,4-diiodobenzene gives 4,4''-bis(2-thienyl)-1,1':4,1''-terphenyl and with 2,5-diiodothiophene, 2,5-bis[4-(2-thienyl)-phenyl]-thiophene is obtained. |
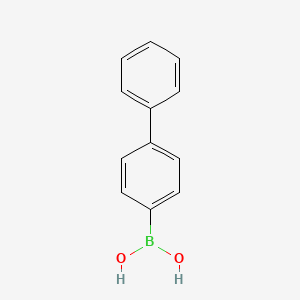
Isomeric SMILES: B(C1=CC=C(C=C1)C2=CC=CC=C2)(O)O
InChIKey: XPEIJWZLPWNNOK-UHFFFAOYSA-N
InChI: InChI=1S/C12H11BO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,14-15H
Here, to extend our knowledge of molecular recognition, we first examined the enhancing effects of four phenylboronic acid compounds other than p-iodophenylboronic acid i.e., 4-biphenylboronic acid, 4-octyloxyphenyl-boronic acid, 3-octyloxyphenylboronic acid, and 4-dodecyloxyphenylboronic acid, for luminol-hydrogen peroxide- horseradish peroxidase reaction in the capillary electrophoresis-chemiluminescence detection system. Only 4-biphenylboronic acid showed an enhancing effect similar to that of p-iodophenylboronic acid; the effect was determined over the range of 0.5 - 10 μM in this system.
The reaction between di-2-thienyl ketone...
As designed, we applied the 4,4'-Biphenylboronic acid mediated method to amplify the weak Raman signal of glucose by testing the strong Raman signal of the reaction product ((…
4-bromo-1,1'-biphenyl

4-biphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1.5h; Inert atmosphere;
With Trimethyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 4.5h;
With water; In tetrahydrofuran; hexane; ethyl acetate;
|
84% |
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 2.5h;
With Triisopropyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃;
|
76% |
|
4-bromo-1,1'-biphenyl; With magnesium; In tetrahydrofuran; for 4h; Heating;
With Trimethyl borate; In tetrahydrofuran; at 0 - 20 ℃;
|
67% |
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In tetrahydrofuran; hexanes; at -80 ℃; Inert atmosphere;
With Trimethyl borate; In tetrahydrofuran; hexanes; at -80 - 20 ℃; Inert atmosphere;
With hydrogenchloride; In tetrahydrofuran; hexanes; water; at 20 ℃; Inert atmosphere;
|
64% |
|
Multistep reaction; (i) Mg, (ii) B(OMe)3, (iii) H2SO4;
|
|
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
With Triisopropyl borate; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 6h;
|
|
|
4-bromo-1,1'-biphenyl; With magnesium; In tetrahydrofuran; at 50 ℃; for 2h;
With Trimethyl borate; In tetrahydrofuran; at -60 - 15 ℃; for 2h; Further stages.;
|
|
|
Multi-step reaction with 2 steps
1.1: n-BuLi / tetrahydrofuran / 0.42 h / -78 °C
1.2: tetrahydrofuran / -78 - 20 °C
2.1: aq. HCl / 3 h / pH 6 - 7
With hydrogenchloride; n-butyllithium; In tetrahydrofuran;
|
|
|
4-bromo-1,1'-biphenyl; With magnesium; In tetrahydrofuran;
With Trimethyl borate;
With hydrogenchloride; Further stages.;
|
|
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In diethyl ether; hexane; toluene; at -64 ℃; for 2.5h;
With Trimethyl borate; In diethyl ether; hexane; toluene; at 20 ℃; for 12.25h;
With hydrogenchloride; water; In diethyl ether; hexane; toluene; at 0 - 10 ℃;
|
|
|
With tetrahydroxydiboron; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N-ethyl-N,N-diisopropylamine; triphenylphosphine; In ethanol; at 20 ℃; for 6h; Inert atmosphere; Sealed tube;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 1 h / -78 - 0 °C / Inert atmosphere
1.2: 12 h / -78 - 20 °C / Inert atmosphere
2.1: hydrogenchloride / tetrahydrofuran / 0.5 h / Inert atmosphere
With hydrogenchloride; n-butyllithium; In tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1.1: magnesium; lithium chloride / Inert atmosphere; Schlenk technique
1.2: Inert atmosphere; Schlenk technique
2.1: copper(I) iodide-lithium chloride / tetrahydrofuran / 20 °C / Schlenk technique; Inert atmosphere
With copper(I) iodide-lithium chloride; magnesium; lithium chloride; In tetrahydrofuran;
|
Triisopropyl borate

4-bromo-1,1'-biphenyl

4-biphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-bromo-1,1'-biphenyl; With n-butyllithium; In diethyl ether; hexane; at -78 ℃; for 0.5h;
Triisopropyl borate; In diethyl ether; hexane; at -78 - 20 ℃;
|
74% |
|
With n-butyllithium; In tetrahydrofuran;
|
1.53 g (90%) |
boric acid tributyl ester
4-biphenylylmagnesium bromide
4-bromo-1,1'-biphenyl
4-iodo-biphenyl
2,4,6-tris([1,1'-biphenyl]-4-yl)boroxine
2-([1,1'-biphenyl]-4-yl)-5,5-dimethyl-1,3,2-dioxaborinane
4-fluoro-biphenyl
(2S,5S)-5-[4-Acetoxy-5-(diethoxy-phosphorylmethyl)-[1,1';4',1'']terphenyl-3-ylmethyl]-2-tert-butyl-3-methyl-4-oxo-imidazolidine-1-carboxylic acid tert-butyl ester
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:762287-57-0
Molecular Formula:C7H8BClO3
Molecular Weight:186.4