Your Location:Home >Products >OLED intermediates >Boric acids >1189047-28-6
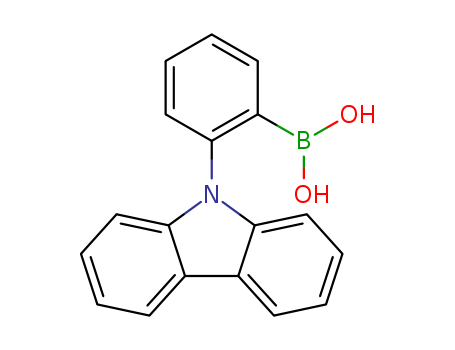

Product Details
Chemical Properties
off-white powder
A series of small molecular isomers, namely o-CzCN, m-CzCN, and p-CzCN, are developed for use as bipolar hosts in blue phosphorescent and fluorescent organic light-emitting diodes (OLEDs). Cyano (CN) substituted phenyl is selected as the n-type unit and N-phenyl-substituted carbazole as the p-type unit. By adjusting the ortho-, meta-, and para-linking styles of the functional units, the physical parameters are regularly tuned to a large extent. The study of complete spatial separation of frontier molecular orbitals and single-carrier devices confirm the bipolar feature. Blue phosphorescent and thermally activated delayed fluorescence (TADF) OLEDs were fabricated using iridium(iii)bis(4,6-(difluorophenyl)pyridinato-N,C2′)picolinate (FIrpic) and 1,2-bis(carbazol-9-yl)-4,5-dicyanobenzene (2CzPN) as doped emitters. A maximum current efficiency of 46.81 cd A-1 and an external quantum efficiency of 23.14% were achieved for the phosphorescent OLED with the m-CzCN host. Furthermore, high efficiencies of 29.23 cd A-1 and 14.98% were obtained for the 2CzPN based blue TADF device with the o-CzCN host, which are higher than the best literature value of 13.6% for 2CzPN devices. Both m-CzCN and o-CzCN always perform better than p-CzCN. The influence of the chemical structures on their properties and performance is interpreted for these CN-decorated host materials.
A compound represented by formula (1) provides an organic electroluminescence device having lifetime further improved: wherein R1 to R9, R11 to R18, R21 to R27, Ar, L1, L2, and L3 are as defined in the description.
A novel compound represented by formula (1): wherein R1 to R7, R11 to R18, L1 to L3, a to c, n, and Ar are as defined in the description, provides an organic electroluminescence device having a device lifetime further improved.
A compound represented by formula (1): wherein R1 to R7, R11 to R18, L1 to L3, a to c, n, and Ar are as defined in the description, provides an organic electroluminescence device having an emission efficiency and a device lifetime further improved.
The invention discloses a pyrimidine derivative and its application, the pyrimidine derivatives having the following -like type (I) indicated by the structure; wherein said R1 , R2 , R3 Are selected from the carbon atom number is 1 - 60 alkyl, substituted and non-substituted aromatic heterocyclic radical, and substituted and non-substituted aromatic ring in base of any one group. The invention through the pyrimidine derivatives key chemical structure and the like is improved, and the pyrimidine derivatives as material is applied to the electro-luminescent layer in the organic electroluminescent device, compared with the prior art can effectively solve the organic electroluminescent device in blue light material of poor stability, low efficiency and the like. (by machine translation)
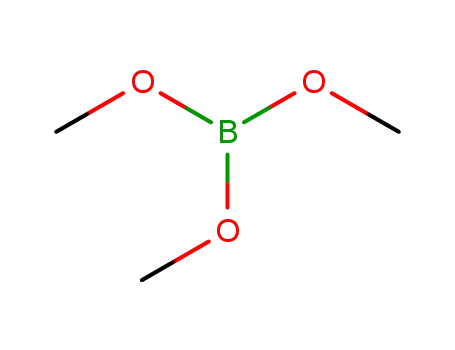
Trimethyl borate

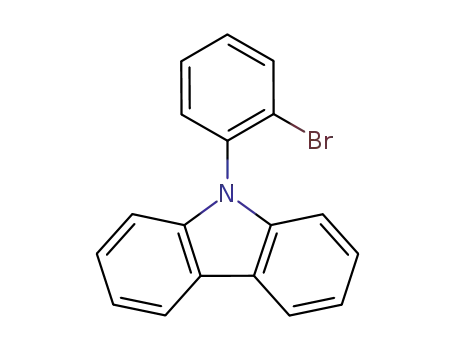
9-(2-bromophenyl)-9H-carbazole

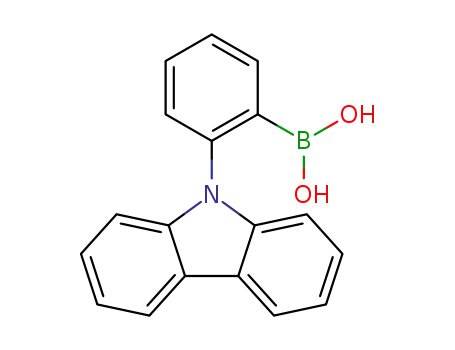
(2-(9H-carbazol-9-yl)phenyl)boronic acid
| Conditions | Yield |
|---|---|
|
9-(2-bromophenyl)-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
|
78% |
|
9-(2-bromophenyl)-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
for 2h;
Inert atmosphere;
Cooling with acetone-dry ice;
Trimethyl borate;
In
tetrahydrofuran; hexane;
for 1h;
Inert atmosphere;
Cooling with acetone-dry ice;
|
69% |
|
9-(2-bromophenyl)-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
for 2h;
Inert atmosphere;
Cooling with acetone-dry ice;
Trimethyl borate;
In
tetrahydrofuran; hexane;
for 1h;
Cooling with acetone-dry ice;
|
69% |
|
9-(2-bromophenyl)-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 4h;
|
43% |
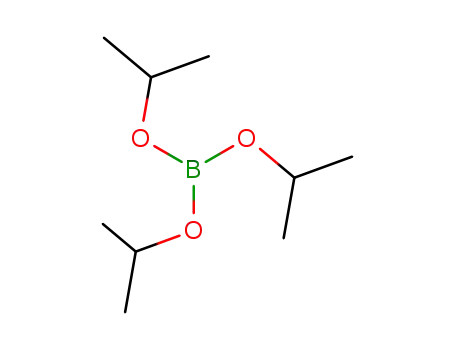
Triisopropyl borate


9-(2-bromophenyl)-9H-carbazole


(2-(9H-carbazol-9-yl)phenyl)boronic acid
| Conditions | Yield |
|---|---|
|
9-(2-bromophenyl)-9H-carbazole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1.5h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 8h;
|
90% |
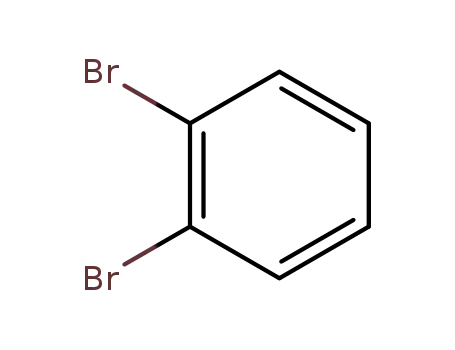
2,3-dibromobenzene
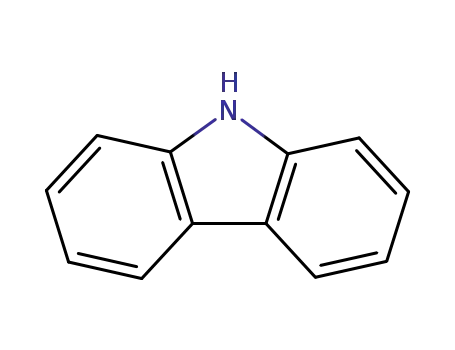
9H-carbazole

9-(2-bromophenyl)-9H-carbazole
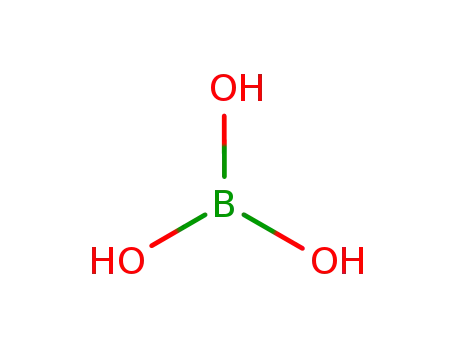
boric acid
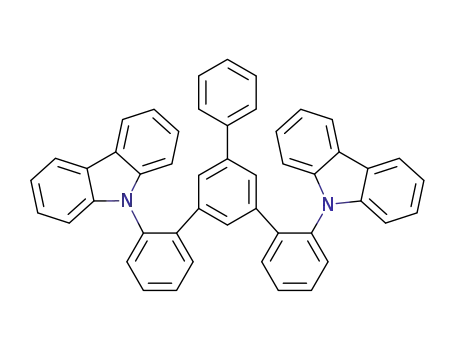
C48H32N2
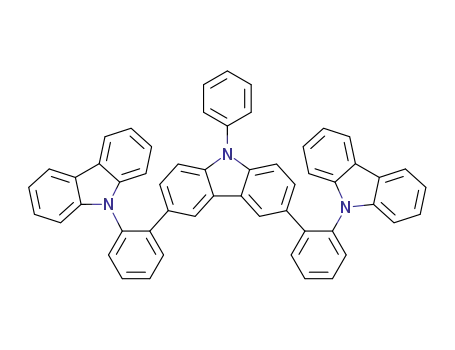
C54H35N3
CAS:355832-04-1
CAS:854952-58-2
CAS:1126522-69-7
Molecular Formula:C24H24BNO2
Molecular Weight:369.3
CAS:444796-09-2