Your Location:Home >Products >OLED intermediates >Boric acids >1126522-69-7

Product Details
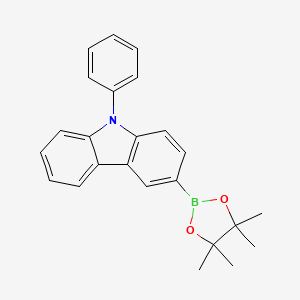
3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-9-phenylcarbazole is Pale yellow powder which is used in medicine.
Isomeric SMILES: B1(OC(C(O1)(C)C)(C)C)C2=CC3=C(C=C2)N(C4=CC=CC=C43)C5=CC=CC=C5
InChIKey: UBASCOPZFCGGAV-UHFFFAOYSA-N
InChI: InChI=1S/C24H24BNO2/c1-23(2)24(3,4)28-25(27-23)17-14-15-22-20(16-17)19-12-8-9-13-21(19)26(22)18-10-6-5-7-11-18/h5-16H,1-4H3
N-phenyl carbazole and triphenylamine functionalized tris(aryl)triazines, as well as the corresponding mononers, have been synthesized by Suzuki cross-coupling reactions. The results are supported by time-dependent density functional theory calculations, delayed and time-resolved fluorescence data.
Provided are the compound represented by Formula 2-K, an organic electric element including a first electrode, a second electrode, and an organic material layer formed between the first electrode and the second electrode, and electronic device thereof, and by including the compound represented by Formula 1 and compound represented by Formula 2 or the compound represented by Formula 2-K in the organic material layer, the driving voltage of the organic electric element can be lowered, and the luminous efficiency and life time of the organic electric element can be improved.
The invention discloses a thiophene ethylene malononitrile structural compound and a preparation method thereof. The structural formula is shown in the specification. The thiophene ethylene malononitrile structural compound is obtained through multi-step
The invention relates to an organic compound based on a triazine and anthrone structure, and applications in OLED devices. The compound has high glass transition temperature and high molecular thermalstability, and is low in absorption in the field of vis
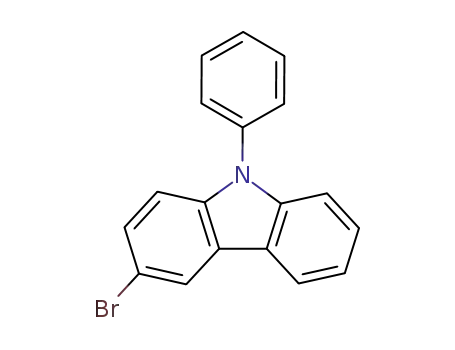
3-bromo-9-phenyl-9H-carbazole

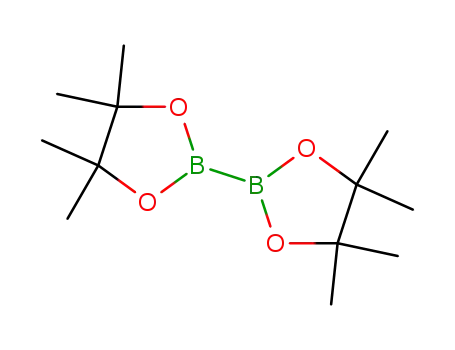
bis(pinacol)diborane

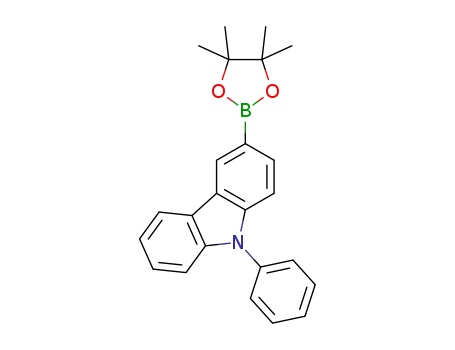
9-phenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
3-bromo-9-phenyl-9H-carbazole; bis(pinacol)diborane; In 1,4-dioxane; for 0.166667h; Inert atmosphere;
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; for 12h; Reflux;
|
95% |
|
With palladium bis[bis(diphenylphosphino)ferrocene] dichloride; potassium acetate; In tetrahydrofuran; at 80 ℃; for 5h; Inert atmosphere;
|
90.5% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In tetrahydrofuran; at 80 ℃; for 5h; Inert atmosphere;
|
90.3% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In tetrahydrofuran; at 80 ℃; for 5h; Inert atmosphere;
|
90.3% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; Inert atmosphere; Reflux;
|
88.4% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90 ℃;
|
85% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90 ℃;
|
84% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90 ℃;
|
84% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 130 ℃; for 12h; Inert atmosphere;
|
81% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; palladium diacetate; In toluene; for 12h; Reflux;
|
81% |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; for 4h; Inert atmosphere; Reflux;
|
80% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 12h; Reflux; Inert atmosphere;
|
75% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 12h; Reflux; Inert atmosphere;
|
75% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; for 24h;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90 ℃;
|
68% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 80 ℃; for 24h; Inert atmosphere;
|
67.3% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 90 ℃;
|
66% |
|
With tris-(dibenzylideneacetone)dipalladium(0); In 1,4-dioxane;
|
39% |
|
With potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,4-dioxane; at 80 ℃; for 6h; Inert atmosphere;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 120 ℃; for 6h;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 130 ℃; for 3h;
|
|
|
With palladium bis[bis(diphenylphosphino)ferrocene] dichloride; potassium acetate; In 1,4-dioxane; at 100 ℃;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 130 ℃; for 3h;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 12h; Reflux;
|
23 g |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In tetrahydrofuran; at 80 ℃; for 5h; Inert atmosphere;
|

3-bromo-9-phenyl-9H-carbazole

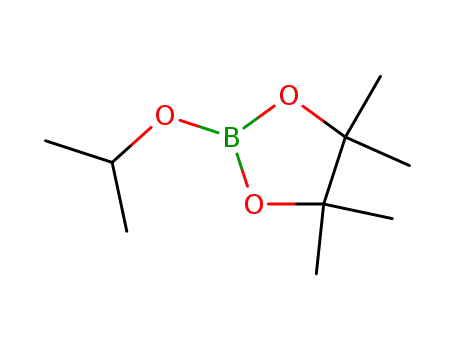
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane


9-phenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
3-bromo-9-phenyl-9H-carbazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; In tetrahydrofuran; hexane; at 20 ℃; for 8h;
|
83% |
|
3-bromo-9-phenyl-9H-carbazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; In tetrahydrofuran; hexane; at -78 - 20 ℃;
|
75% |
|
3-bromo-9-phenyl-9H-carbazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 0.5h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; In tetrahydrofuran; at -78 - 20 ℃;
|
68% |
|
3-bromo-9-phenyl-9H-carbazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 2h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; In tetrahydrofuran; at 20 ℃;
|
65% |
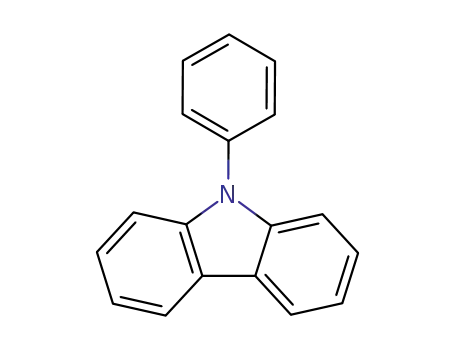
N-phenylcarbazole
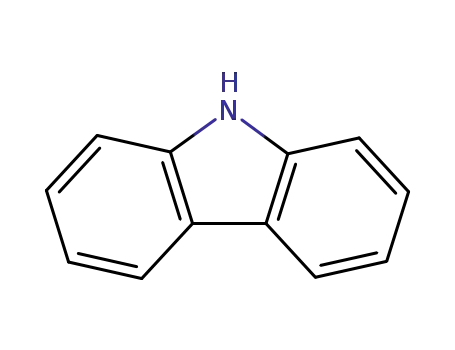
9H-carbazole

3-bromo-9-phenyl-9H-carbazole

bis(pinacol)diborane

N-phenylcarbazole
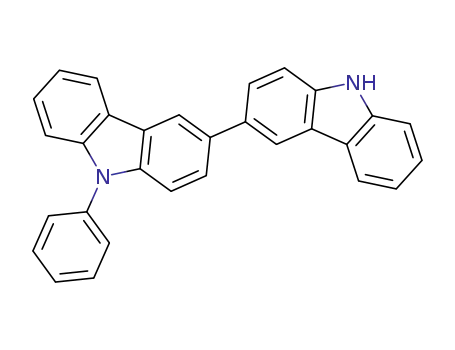
9-phenyl-3,3'-bicarbazole

9-(dibenzo[b,d]thiophen-2-yl)-9'-phenyl-9H,9'H-3,3'-bicarbazole

9-(dibenzo[b,d]thiophen-4-yl)-9?-phenyl-9H,9'H-3,3?-bicarbazole
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:1189047-28-6