Your Location:Home >Products >Functional intermediates >300-57-2

Product Details
Chemical Properties
colourless liquid
Chemical Properties
Allylbenzene is a colorless, flammable chemical, incompatible with strong oxidizing agents. On decomposition, allylbenzene releases carbon monoxide and carbon dioxide.
Uses
Allylbenzene is used in organic synthesis.
Synthesis Reference(s)
Journal of the American Chemical Society, 95, p. 7777, 1973 DOI: 10.1021/ja00804a039
Health Hazard
Exposures to allylbenzene cause irritation to the skin, eyes, and respiratory system. It causes harmful effects if inhaled or swallowed and causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
storage
Allylbenzene should be kept stored in a cool, dry, well-ventilated area with adequate ventilation during use.
Precautions
Workers while handling allylbenzene should not breathe dust or vapor, or ingest the chemical substance. Allylbenzene should only be used in a chemical fume hood and sources of ignition should be avoided. Workers should wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA protection regulations.
InChI:InChI=1/C9H10/c1-2-6-9-7-4-3-5-8-9/h2-8H,1H3/b6-2+
Herein, we report a ring-opening 1,3-arylboration of aryl cyclopropanes using BCl3 in the presence of arene nucleophiles. Formal 1,3-oxy arylation and 1,3-amino arylation of the arylcyclopropane via one-pot derivatization of the installed boron group were also achieved.
Highly symmetric [Pd3]+ clusters that present delocalized metal-metal bonds can catalyse the selective semi-reduction of internal alkynes to cis-alkenes. Studies on factors governing the formation of all-metal aromatics enabled the design of an optimised catalytic system that delivers cis-alkenes with almost complete selectivity on a gram scale with very low catalyst loadings (0.03 mol%).
-
Graphene was successfully modified with gold nanoparticles in a facile route by reducing chloroauric acid in the presence of sodium dodecyl sulfate, which is used as both a surfactant and reducing agent. The gold nanoparticles-graphene hybrids were characterized by high-resolution transmission electron microscopy, atomic force microscopy, X-ray photoelectron spectroscopy, Raman spectroscopy, X-ray diffraction and energy X-ray spectroscopy. We demonstrate for the first time that the gold nanoparticles-graphene hybrids can act as efficient catalysts for the Suzuki reaction in water under aerobic conditions. The catalytic activity of gold nanoparticles-graphene hybrids was influenced by the size of the gold nanoparticles.
Water soluble palladium(II) complexes of the type PdCl2(phosphine)2 with sulfonated phosphines as ligands catalyse the reductive dehalogenation of allyl or benzyl halogenides by means of formates in a biphasic water/heptane system.This reaction can be promoted by addition of polyethers of different types as phase transfer catalysts.Enhancement of reaction rate and control of selectivity are investigated in the reductive conversion of (E)-Ph-CH=CHCH2Cl leading to (E)-Ph-CH=CH-CH3 and Ph-CH2-CH=CH2.
An allylarylation of electron-deficient alkenes with aryl boronates and allylic carbonates has been developed. This method allows access to a wide variety of carbon skeletons from readily available starting materials. Mechanistic studies indicate that this reaction is enabled by a cooperative catalysis based on merging Pd0/PdII redox and PdII/PdII non-redox catalytic cycles.
-
A borate-containing caged triarylphosphine L-X (X = Na or NBu4), featuring a 9-phospha-10-boratriptycene framework, was synthesized and characterized by NMR spectroscopy and X-ray diffraction analysis. The NMR coupling constant of the corresponding phosphine selenide indicated a higher electron-donating property of the borate-phosphine L compared to that of the 9-phospha-10-silatriptycene derivative (Ph-TRIP). The coordination property of L-X to [PdCl(η3-allyl)]2 was dependent on the countercation, giving a neutral Pd complex [PdCl(η3-allyl)(L-NBu4)] from L-NBu4 in CH2Cl2 or a zwitterionic Pd complex [Pd(η3-allyl)(MeCN)(L)] from L-Na in MeCN/CH2Cl2. Utility of L-X as a ligand for metal catalysis was demonstrated in the Pd-catalyzed Suzuki-Miyaura cross-coupling of aryl chlorides.
The mechanism of the reaction of allyl complexes 3-2-R'C3H4)(N-N')>+ (N-N' = α-diimine ligand) with BPh4- in the presence of activated olefins (ol), yielding the products 2-ol)(N-N')> and PhCH2C(R')=CH2, has been investigated.The results are interpreted in terms of extensive association between the cationic substrate and the BPh4- anion in a tight ion-pair, followed by rate-determining phenyl transfer to the palladium center and fast reductive elimination of allylbenzene.
-
Dehydrohalogenation, or elimination of hydrogen-halide equivalents, remains one of the simplest methods for the installation of the biologically-important olefin functionality. However, this transformation often requires harsh, strongly-basic conditions, rare noble metals, or both, limiting its applicability in the synthesis of complex molecules. Nature has pursued a complementary approach in the novel vitamin B12-dependent photoreceptor CarH, where photolysis of a cobalt-carbon bond leads to selective olefin formation under mild, physiologically-relevant conditions. Herein we report a light-driven B12-based catalytic system that leverages this reactivity to convert alkyl electrophiles to olefins under incredibly mild conditions using only earth abundant elements. Further, this process exhibits a high level of regioselectivity, producing terminal olefins in moderate to excellent yield and exceptional selectivity. Finally, we are able to access a hitherto-unknown transformation, remote elimination, using two cobalt catalysts in tandem to produce subterminal olefins with excellent regioselectivity. Together, we show vitamin B12to be a powerful platform for developing mild olefin-forming reactions.
The epoxidation of olefin as a strategy to protect carbon-carbon double bonds is a well-known procedure in organic synthesis, however the reverse reaction, deprotection/deoxygenation of epoxides is much less developed, despite its potential utility for the synthesis of substituted olefins. Here, we disclose a clean protocol for the selective deprotection of epoxides, by combining commercially available organophosphorus ligands and gold nanoparticles (Au NP). Besides being successfully applied in the deoxygenation of epoxides, the discovered catalytic system also enables the selective reduction N-oxides and sulfoxides using molecular hydrogen as reductant. The Au NP catalyst combined with triethylphosphite P(OEt)3 is remarkably more reactive than solely Au NPs. The method is not only a complementary Au-catalyzed reductive reaction under mild conditions, but also an effective procedure for selective reductions of a wide range of valuable molecules that would be either synthetically inconvenient or even difficult to access by alternative synthetic protocols or by using classical transition metal catalysts. This journal is
Two strategies were reported to prevent the deactivation of Frustrated Lewis pairs (FLPs) in the hydrogenation of terminal alkynes: reducing the Lewis acidity and polymerizing the Lewis acid. A polymeric Lewis acid (P-BPh3) with high stability was designed and synthesized. Excellent conversion (up to 99%) and selectivity can be achieved in the hydrogenation of terminal alkynes catalyzed by P-BPh3. This catalytic system works quite well for different substrates. In addition, the P-BPh3 can be easily recycled.
We report here a mechanistically distinct tactic to carry E2-type eliminations on alkyl halides. This strategy exploits the interplay of α-aminoalkyl radical-mediated halogen-atom transfer (XAT) with desaturative cobalt catalysis. The methodology is high-yielding, tolerates many functionalities, and was used to access industrially relevant materials. In contrast to thermal E2 eliminations where unsymmetrical substrates give regioisomeric mixtures, this approach enables, by fine-tuning of the electronic and steric properties of the cobalt catalyst, to obtain high olefin positional selectivity. This unprecedented mechanistic feature has allowed access tocontra-thermodynamic olefins, elusive by E2 eliminations.
propargyl benzene

styrene

allylbenzene

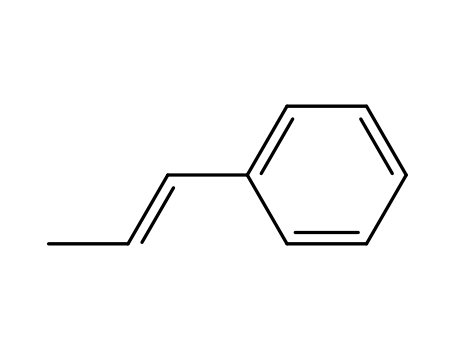
1-propenylbenzene

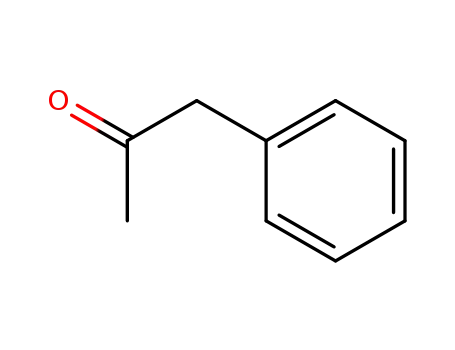
1-phenyl-acetone
| Conditions | Yield |
|---|---|
|
With
water;
RuCl2(C6H6)(PPh2(C6F5));
for 12h;
Further byproducts given. Title compound not separated from byproducts;
|
21.7 % Chromat. 19.3 % Chromat. 34 % Chromat. 6.2 % Chromat. |
|
With
water;
|
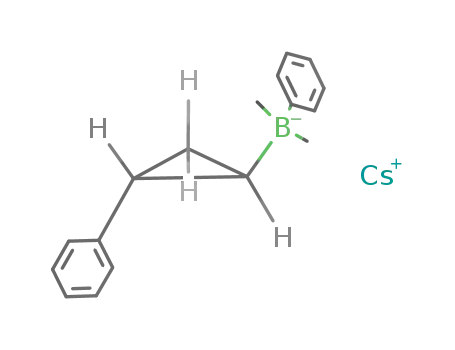
cesium phenyl(2-phenylcyclopropyl)dimethylborate


styrene


allylbenzene

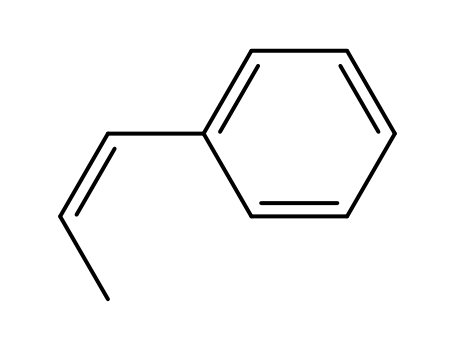
cis-1-phenyl-1-propylene

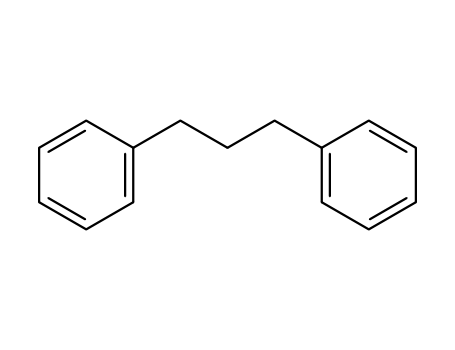
1,3-diphenylpropane

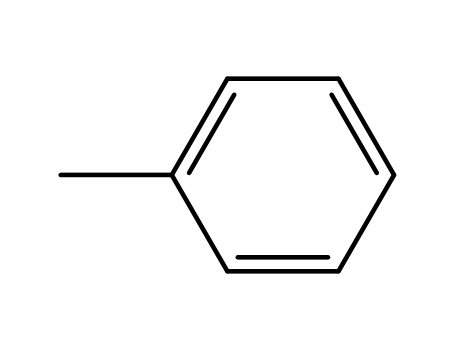
toluene
| Conditions | Yield |
|---|---|
|
With
methanol;
In
tetrahydrofuran;
Irradiation (UV/VIS); Further Products. Purging of a THF soln. of Cs-salt with N2, addn. of MeOH in THF, irradn. at 254 nm.; Monitored by (11)B-NMR and GC.;
|
25% 2% 1% 8% 6% |
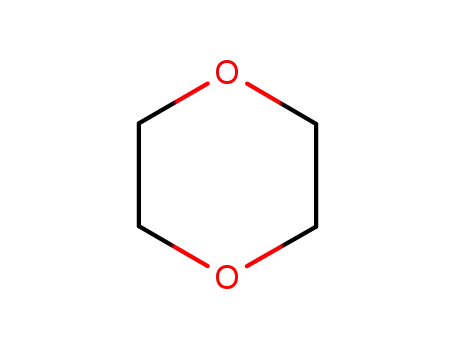
1,4-dioxane
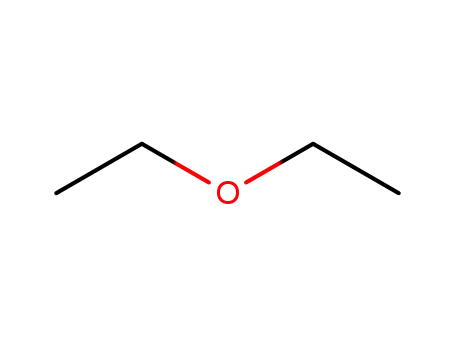
diethyl ether
allyl octyl ether
diphenylmagnesium
3-(3-phenylprop-2-enyl)dihydro-2,5-furandione
dichloro-methyl-(3-phenyl-propyl)-silane
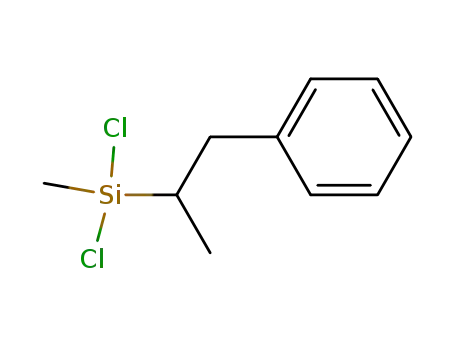
dichloro-methyl-(1-methyl-2-phenyl-ethyl)-silane
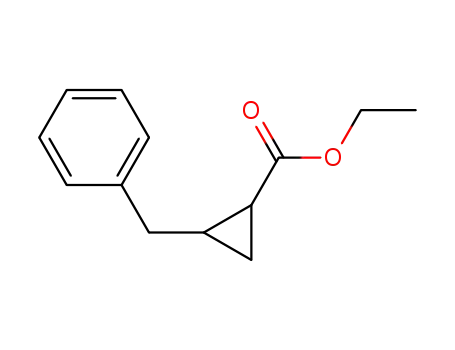
2-benzylcyclopropanecarboxylic acid ethyl ester
CAS:69227-47-0
CAS:27813-21-4
CAS:1746-13-0