Your Location:Home >Products >Functional intermediates >1746-13-0
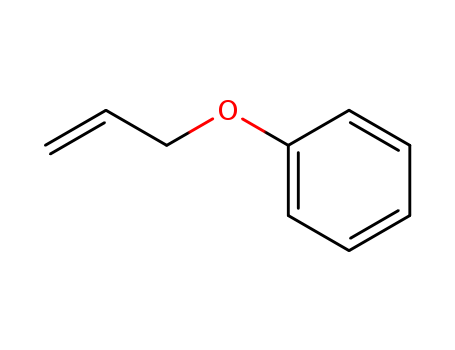

Product Details
Chemical Properties
CLEAR COLOURLESS TO VERY SLIGHTLY YELLOW LIQUID
Uses
Allyl phenyl ether is an pharmaceutical and OLED intermediate.
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 2705, 1959 DOI: 10.1021/ja01520a030Synthetic Communications, 23, p. 2527, 1993 DOI: 10.1080/00397919308012585Tetrahedron Letters, 32, p. 6315, 1991 DOI: 10.1016/0040-4039(91)80156-Z
InChI:InChI=1/C9H10O/c1-2-8-10-9-6-4-3-5-7-9/h2-7H,1,8H2
-
The complexes trans- (R=Ph, 1; or C6H4Me-p, 2) and trans- 3 have been prepared by reactions of trans- or trans- with allyl aryl sulphides.Complex 1 reacts with various organic halides such as allyl chloride, benzyl bromide and methyl iodide to give allyl phenyl sulphide and methyl phenyl sulphide, respectively; trans- (X=Cl, 4; B3, 5; or I, 6) were isolated from the reaction mixtures.The complexe trans- also reacts with allyl chloride to give allyl phenyl ether together with complex 4.The structure of complex 5 has been determined by X-ray crystallography: orthorhombic, space group Pbca with a=12.306(3), b=20.078(5), c=11.753(2) Angstroem, Z=8, R=0.036 and R'=0.042.It has a square-planar co-ordination around the palladium centre.The reaction of allyl chloride with complex 1 in toluene obeys first-order kinetics in the concentrations of both allyl chloride and 1.
The activation of potassium fluoride for nucleophilic fluorination of alkyl halides is an important challenge because of the high lattice energy of this salt and its low solubility in many polar aprotic solvents. Crown ethers have been used for increasing the solubilization of KF during several decades. Nevertheless, these macrocycles are not enough to produce a high reaction rate. In this work, theoretical methods were used for designing a synergic combination of bulky diols with crown ethers able to accelerate this kind of reaction. The calculations have predicted that the bulky diol 1,4-Bis(2-hydroxy-2-propyl)benzene, which has distant hydroxyl groups, is able to catalyze nucleophilic fluorination in combination with 18-crown-6 via two hydrogen bonds to the SN2 transition state. Experimental studies following the theoretical predictions have confirmed the catalytic effect and the estimated kinetic data point out that the bulky diol at 1 mol L-1 in combination with 18-crown-6 is able to produce an 18-fold increase in the reaction rate in relation to crown ether catalysis only. The reaction produces 46% yield of fluorination after 24 h at moderate temperature of 82 °C, with minimal formation of the side elimination product. Thus, this work presents an improved method for fluorination with KF salt.
A Pd-bisphosphine complex and several organic functionalities were immobilized on the same SiO2 surface. The samples thus prepared were characterized by solid-state NMR, XPS, and XAFS. Based on the curve-fitting analysis of Pd K-edge EXAFS spectra, both the local environment of the immobilized Pd complexes and the interactions with the co-immobilized organic functions were discussed. The SiO2-supported Pd-bisphosphine complex and DABCO exhibited excellent catalytic performance for the allylation of various nucleophiles: a TON of up to 106000 was obtained. Both the catalyst activation pathway and the reaction mechanism were also discussed on the basis of the structure of the used catalyst samples.
Provided is a method for producing (alkyl)arene compounds represented by Formulae 3-1, 3-2, and 3-3 by the Friedel-Crafts alkylation reaction of alkyl halide compounds and arene compounds using organic phosphine compounds as a catalyst.
Desulfurization of a variety of thiiranes to alkenes occurs chemoselectively in high yields upon treatment with MoCl5/Zn system under mild conditions. The new methodology demonstrates high functional group tolerance toward chloro, bromo, fluoro, methoxy, ester, ether and keto groups.
The zinc salt-catalyzed reduction of α-aryl imino esters, diketones and phenylacetylenes with water as hydrogen source and zinc as reductant was successfully conducted. The presented method provides a low-cost, environmentally friendly and practical preparation of α-aryl amino esters, α-hydroxyketones and phenylethylenes. By using D2O as deuterium source, the corresponding products were obtained in high efficiency with excellent deuterium incorporation rate, which gives a cheap and safe tool for access to valuable deuterium-labelled compounds. This journal is
The invention discloses a preparation method of 3 -phenoxybromopropane or an analogue thereof, wherein 3 - phenoxybromopropane and an allyl compound thereof are obtained through substitution reaction and addition reaction so as to avoid the inconvenience of using gaseous hydrogen bromide, 2nd-step addition reaction is realized by using the brominated salt and the acid in situ, and the process is simple in operation. The condition is easy to control, the atom economy is good, the aspect of environmental impact is low pollution, zero emission accords with the current green chemical synthesis direction, and the cost is economic.
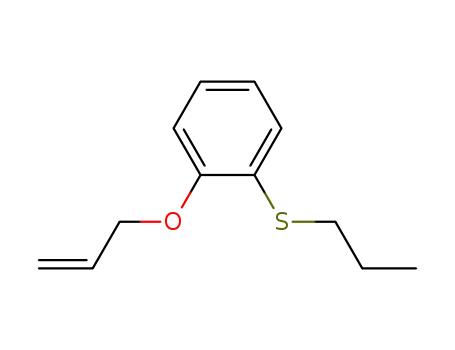
2-allyloxy-S-propylthiophenol

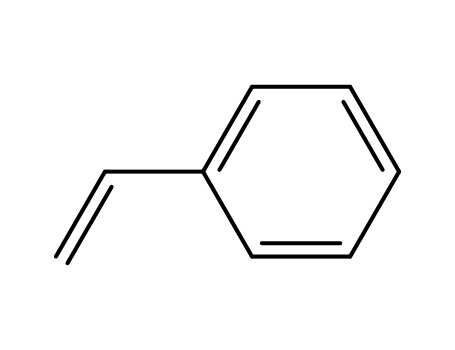
styrene

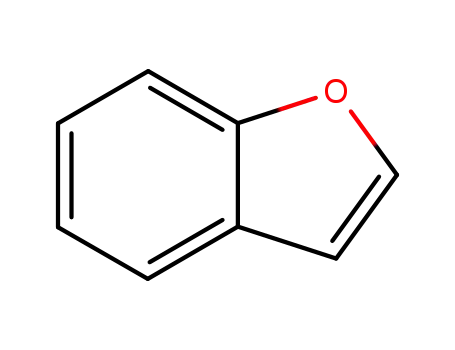
1-benzofurane

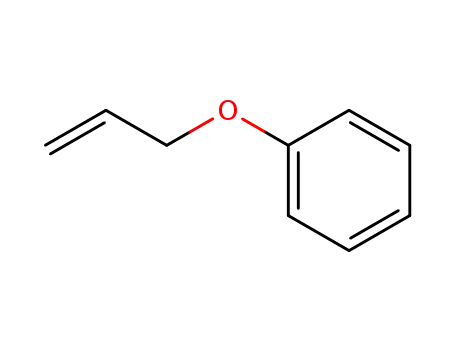
allyl phenyl ether

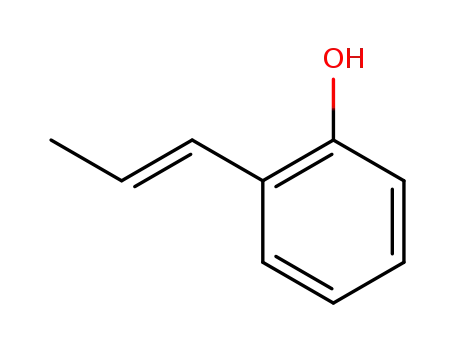
(E)-2-propenylphenol

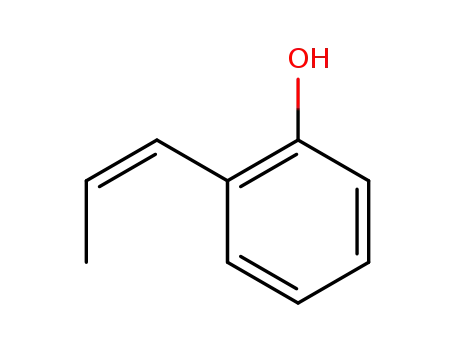
β-methyl-vinylphenol(Z)-2-(prop-1-en-1-yl)phenol
| Conditions | Yield |
|---|---|
|
at 750 ℃;
for 0.333333h;
under 0.001 Torr;
Mechanism;
pyrolysis of o-substituted N-, O-, and S-allyl compounds;
|
12 % Chromat. 9 % Chromat. 15 % Chromat. |
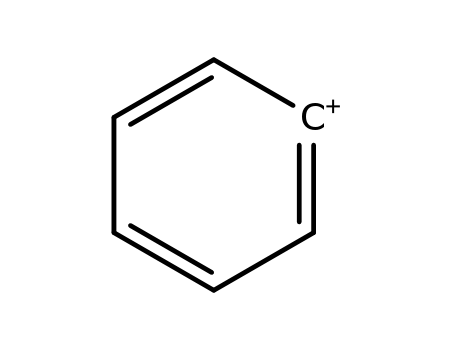
phenylium

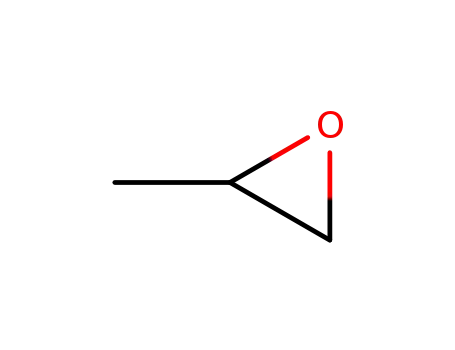
methyloxirane

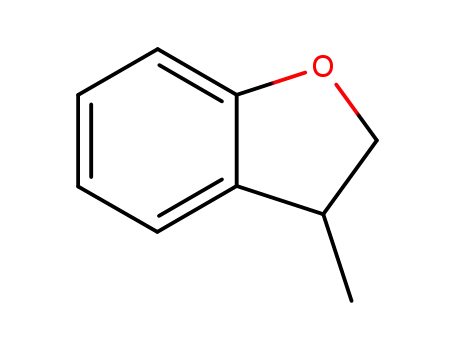
3-methyl-2,3-dihydrobenzofuran

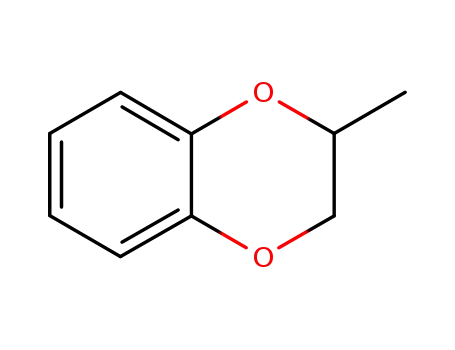
2-methylbenzo-1,4-dioxane


allyl phenyl ether

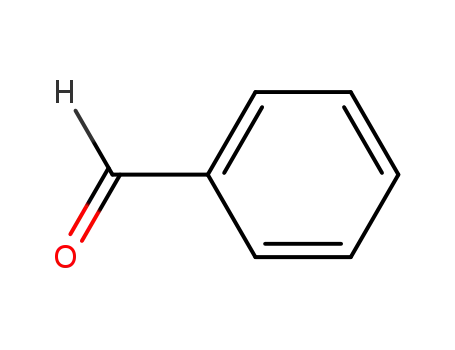
benzaldehyde
| Conditions | Yield |
|---|---|
|
With
helium; oxygen;
for 8760h;
under 684 Torr;
Further byproducts given;
Ambient temperature;
dark;
|
26 % Chromat. 26 % Chromat. 18 % Chromat. 12 % Chromat. |
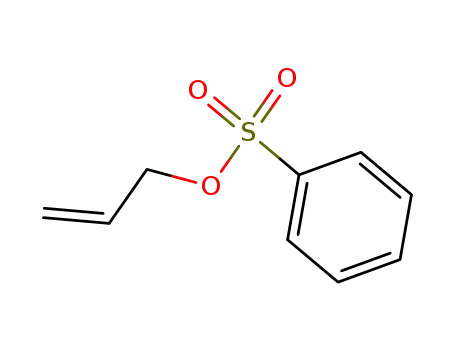
allyl benzenesulfonate
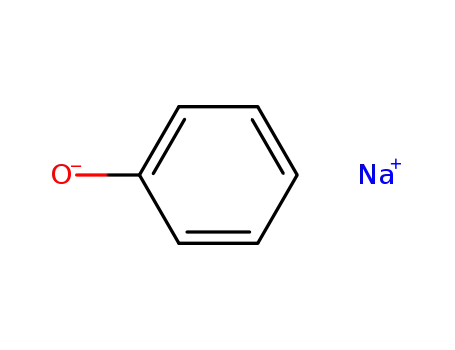
sodium phenoxide
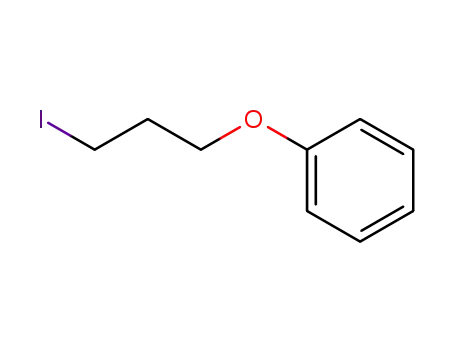
1-(3-iodopropoxy)benzene
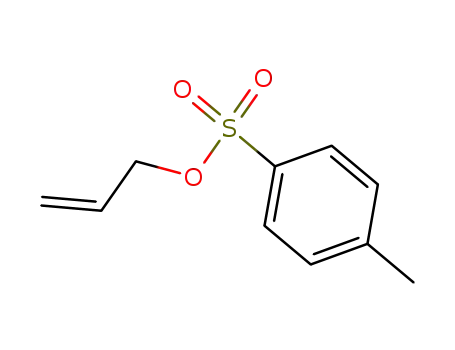
allyl tosylate
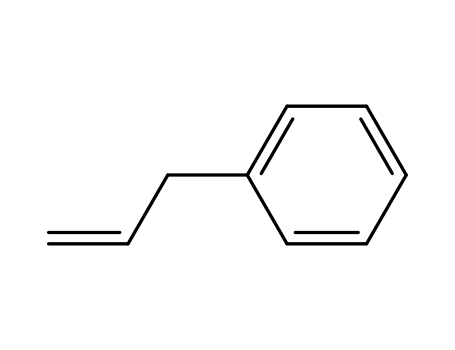
allylbenzene
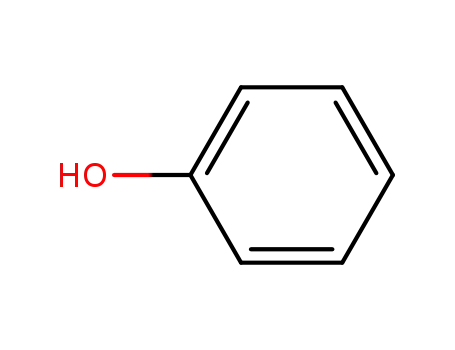
phenol
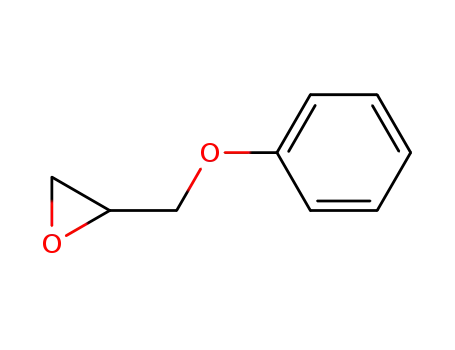
Phenyl glycidyl ether
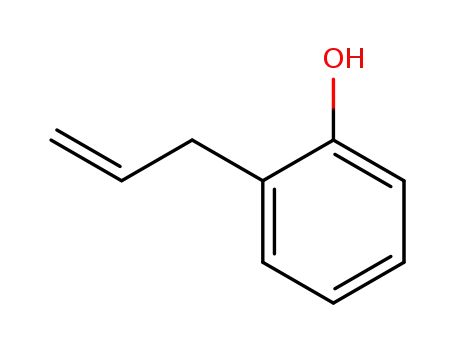
o-Allylphenol
CAS:15133-82-1
CAS:300-57-2