Your Location:Home >Products >OLED intermediates >Carbazoles >56525-79-2

Product Details
The 3,6-diphenylcarbazole thus represent an interesting scaffold to develop antitumor agents interacting with nucleic acids. 3,6-diphenylcarbazole is a weak donor for TADF properties. 3,6-diphenylcarbazole (PhCz) derivatives had better CT properties because of the stronger donor nature.
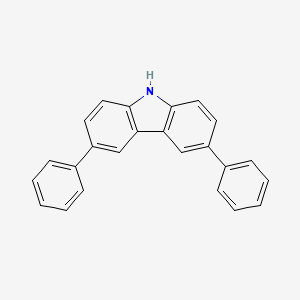
Isomeric SMILES: C1=CC=C(C=C1)C2=CC3=C(C=C2)NC4=C3C=C(C=C4)C5=CC=CC=C5
InChIKey: PCMKGEAHIZDRFL-UHFFFAOYSA-N
InChI: InChI=1S/C24H17N/c1-3-7-17(8-4-1)19-11-13-23-21(15-19)22-16-20(12-14-24(22)25-23)18-9-5-2-6-10-18/h1-16,25H
3,6-di-tert-butyl-9H-carbazole and 3,6-diphenyl-9H-carbazole have been widely used to construct blue thermally activated delayed fluorescence (TADF) emitters. However, it is difficult for related emitters to achieve maximum external quantum efficiency (EQEmax) above 20 % with the Commission Internationale de l'Eclairage (CIE) y ≤ 0.12.
A proof-of-the-concept TADF emitter with 3,6-diphenyl-9H-carbazole (D) connected at the 3’3-positions of 9H-xanthen-9-one (A), the void carbon-atom with no distribution of the highest …
The HOMOs of the three compounds are mainly distributed on the electron-donating moieties of 3,6-diphenylcarbazole (PCz) and carbazole (Cz), … 3. Nucleophilic aromatic substitution of …
3,6-dibromo-9H-carbazole

phenylboronic acid

3,6-diphenylcarbazole
| Conditions | Yield |
|---|---|
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 90 ℃;
|
86% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; at 90 ℃; for 24h; Inert atmosphere;
|
86% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In ethanol; water; toluene; at 80 ℃; for 20h; Inert atmosphere;
|
83% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 16h; Inert atmosphere;
|
76% |
|
With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; tris-(dibenzylideneacetone)dipalladium(0); potassium phosphate monohydrate; In water; toluene; Inert atmosphere; Reflux;
|
75% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 120 ℃;
|
75.5% |
|
With potassium carbonate; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; Inert atmosphere; Reflux;
|
74% |
|
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In toluene; at 80 ℃; for 8h; Inert atmosphere;
|
74% |
|
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In toluene; at 80 ℃; for 8h; Inert atmosphere;
|
74% |
|
With potassium carbonate; tetrakis(triphenylphosphine) palladium(0); In toluene; for 10h; Heating / reflux;
|
73% |
|
With palladium diacetate; potassium carbonate; triphenylphosphine; In tetrahydrofuran; water; Reflux;
|
69% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃;
|
68% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃;
|
68% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In ethanol; water; toluene; for 12h; Inert atmosphere; Reflux;
|
64% |
|
With potassium carbonate; palladium diacetate; tris-(o-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 80 ℃; for 3.5h;
|
63% |
|
With potassium carbonate; palladium diacetate; Tri(p-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 80 ℃; for 3.5h;
|
63% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100 ℃; for 17h; Inert atmosphere;
|
63% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100 ℃; for 16h;
|
63% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 10h; Inert atmosphere;
|
48% |
|
With tetrakis(triphenylphosphine) palladium(0); caesium carbonate; In ethanol; water; toluene; at 100 ℃; for 16h; Inert atmosphere; Schlenk technique;
|
46% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In water; N,N-dimethyl-formamide; at 80 ℃; for 4h; Inert atmosphere;
|
33% |
|
With sodium carbonate; tetrakis(triphenylphosphine) palladium(0); In 1,2-dimethoxyethane; ethanol; water; for 18h; Heating / reflux;
|
|
|
|
|
|
With sodium carbonate; tetrakis(triphenylphosphine) palladium(0); In ethanol; toluene;
|
|
|
3,6-dibromo-9H-carbazole; phenylboronic acid; With sodium carbonate; In 1,4-dioxane; water; for 1h; Inert atmosphere;
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; In 1,4-dioxane; water; Reflux; Inert atmosphere;
|
38 g |
|
With tetrakis(triphenylphosphine) palladium(0); tetrabutylammomium bromide; potassium carbonate; In toluene;
|
3,6-diiodocarbazole

phenylboronic acid

3,6-diphenylcarbazole
| Conditions | Yield |
|---|---|
|
With barium dihydroxide; tris-(o-tolyl)phosphine; palladium diacetate; In 1,2-dimethoxyethane; water; at 80 ℃; for 8h;
|
96% |
|
With palladium diacetate; barium dihydroxide; tris-(o-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 60 ℃;
|
96% |
|
With barium hydroxide octahydrate; palladium diacetate; tris-(o-tolyl)phosphine; In 1,2-dimethoxyethane; water; at 80 ℃; for 8h; Inert atmosphere;
|
84% |
3,6-diiodocarbazole
phenylboronic acid
3,6-dibromo-9H-carbazole
1,2-dimethoxyethane
1,8-diiodo-3,6-diphenyl-carbazole
C32H31N3O4
C36H39N3O4
C36H39N3O4
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:6299-16-7