Your Location:Home >Products >OLED intermediates >Carbazoles >37500-95-1
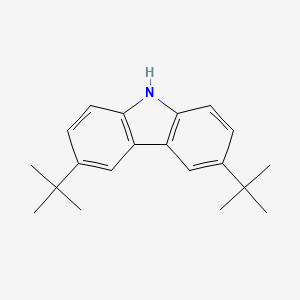

Product Details
|
Chemical Properties |
gray powder |
|
Uses |
3,6-Di-tert-butylcarbazole is mainly used as a monomeric precursor in the syntheses of new carbazole based materials which consist of ethynylphenyl. These materials include 9-(4-bromophenyl)-3,6-di-tert-butylcarbazol and 2-(4-(2-(4-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)ethynyl)benzylidene)malononitrile (PBM) which can be further be used in organic light emitting diodes (OLEDs) and optical switching devices. |
|
General Description |
3,6-Di-tert-butylcarbazole is a carbazole based material with hole transporting characteristics. The 3,6-Di-tert-butyl component of the carbazole results in an increase in the glass transition temperature (Tg) of the compound. It can be used in combination with another carbazole to form novel electroluminescent materials. |
InChI=1S/C20H25N/c1-19(2,3)13-7-9-17-15(11-13)16-12-14(20(4,5)6)8-10-18(16)21-17/h7-12,21H,1-6H3
1,3-Bis(pentafluorophenyl-imino)isoindol...
Three cationic iridium complexes contain...
This article reports the synthesis of ne...
Macrocycles built of carbazole and pyrid...
Two new analogues of a popular host mate...
Novel starburst triazatruxenes functiona...
Circularly polarized organic light-emitt...
An ideal host material with high triplet...
A group of dendrimers with oligo-carbazo...
A novel carbazole-containing porphyrinoi...
A combination of electrochemical, spectr...
A series of novel carbazole-based materi...
Carboxylate sensing solid-contact ion-se...
A series of carbazolyl-substituted quina...
A new charge-neutral Ru(iii) complex RuL...
A series of new D-D-π-A-type organic dye...
Herein we report the optimised synthesis...
Two TADF dendrimers composed of diphenyl...
(Graph Presented) A new class of highly ...
Synthesis of fused heterocyclic aldehyde...
Carbazole dendrimers up to 4th generatio...
A new bipolar host material t3Cz-SO was ...
The spectral and photophysical propertie...
A series of fused heterocycles possessin...
The invention provides a process to form...
Cyclometalated Ir(III) complexes are oft...
Two highly emissive carbazole-containing...
tertiary butyl chloride

9H-carbazole

3,6-di(tert-butyl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 8h;
|
95% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h;
|
94% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 3h; Inert atmosphere; Sonication;
|
92% |
|
With zinc(II) chloride; In nitromethane; for 16h; Inert atmosphere;
|
86% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h; Inert atmosphere;
|
85% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h; Inert atmosphere;
|
85% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 12h; Inert atmosphere;
|
85% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 24h;
|
83% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 6h; Inert atmosphere;
|
81% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h; Inert atmosphere;
|
81% |
|
With aluminum (III) chloride; In nitromethane; at 20 ℃; Inert atmosphere; Schlenk technique;
|
79% |
|
With zinc(II) chloride; Inert atmosphere;
|
77% |
|
In nitromethane; at 20 ℃; for 8h; Inert atmosphere;
|
76% |
|
With nitromethane; zinc(II) chloride; for 15.33h; Glovebox; Inert atmosphere;
|
75% |
|
With aluminum (III) chloride; In dichloromethane; at 25 ℃; for 24h;
|
75% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h; Inert atmosphere;
|
73% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 12h; Inert atmosphere; Schlenk technique;
|
70% |
|
With zinc(II) chloride; In nitromethane; Inert atmosphere;
|
70% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 24h;
|
69% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 16h; Inert atmosphere;
|
66% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 10h;
|
65% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃;
|
61% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃;
|
60.6% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 10.3333h; Cooling with ice;
|
60% |
|
9H-carbazole; With zinc(II) chloride; In nitromethane; at 20 ℃; for 0.166667h;
tertiary butyl chloride; In nitromethane; for 5h;
|
55% |
|
With aluminium trichloride; In dichloromethane; at 0 - 20 ℃; for 24h;
|
54% |
|
With aluminum (III) chloride; at 20 ℃; for 24h;
|
54% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃;
|
54% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 9h;
|
54% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃;
|
54% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 24h;
|
54% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 24h;
|
51% |
|
With aluminum (III) chloride;
|
50% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 6h; Inert atmosphere;
|
50% |
|
With aluminium trichloride; In dichloromethane; at 20 ℃; for 16h;
|
47% |
|
With zinc(II) chloride; In nitromethane; Inert atmosphere;
|
46% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 24h; Inert atmosphere;
|
45% |
|
With aluminum (III) chloride; In chloroform; at 0 ℃; for 12h;
|
40% |
|
9H-carbazole; With zinc(II) chloride; In nitromethane; at 20 ℃; Inert atmosphere;
tertiary butyl chloride; In nitromethane; at 20 ℃; for 5h;
|
40% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h; Inert atmosphere;
|
39% |
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 48h;
|
37.4% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 168h; Inert atmosphere;
|
36% |
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; Inert atmosphere;
|
35% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 20.5h;
|
32% |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 20.5h; Inert atmosphere;
|
32% |
|
With zinc(II) chloride; In nitromethane; for 5h;
|
11% |
|
With zinc(II) chloride; In nitromethane; for 5h;
|
11% |
|
With aluminium trichloride;
|
|
|
With zinc(II) chloride; In nitromethane; at 40 - 50 ℃; for 5h;
|
|
|
With aluminium trichloride;
|
|
|
With aluminium trichloride; In dichloromethane;
|
|
|
With aluminium trichloride;
|
|
|
With zinc(II) chloride; In nitromethane; at 40 - 50 ℃;
|
|
|
With aluminum (III) chloride; at 20 ℃; for 24h;
|
|
|
With zinc(II) chloride;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; Inert atmosphere;
|
|
|
With zinc(II) chloride; In nitromethane; at 20 ℃; Molecular sieve; Inert atmosphere;
|
64.5 mg |
|
With N-Bromosuccinimide;
|
|
|
With zinc(II) chloride; In nitromethane; Inert atmosphere; Schlenk technique;
|
|
|
With aluminum (III) chloride; Inert atmosphere;
|
|
|
With aluminum (III) chloride; In dichloromethane;
|
|
|
With aluminum (III) chloride;
|
|
|
With aluminum (III) chloride; In nitromethane; at 20 ℃; for 24h;
|
|
|
With nitromethane; zinc(II) chloride; at 20 ℃; for 5h; Inert atmosphere;
|
|
|
With aluminum (III) chloride; In dichloromethane; Inert atmosphere;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 24h;
|
|
|
With aluminum (III) chloride;
|
|
|
With aluminum (III) chloride; In dichloromethane;
|
|
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h;
|
|
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 5h;
|
|
|
With aluminum (III) chloride;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 20 ℃; for 24h;
|
|
|
With aluminum (III) chloride; In chloroform; for 16h;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; for 24h; Inert atmosphere;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 0 ℃; for 12h;
|
|
|
With zinc(II) chloride; In nitromethane; at 20 ℃;
|
|
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 24h;
|
|
|
With aluminum (III) chloride; In dichloromethane; at 0 - 20 ℃; Inert atmosphere;
|
9.6 g |
|
With zinc(II) chloride; In nitromethane; at 20 ℃; for 24h;
|
|
|
With zinc(II) chloride; In nitromethane; dichloromethane; for 0.5h; Sonication;
|
2,2'-bis(acetamido)-5,5'-di-tert-butylbiphenyl

3,6-di(tert-butyl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With phosphoric acid; In diethylene glycol; at 200 ℃; for 24h;
|
74% |
tertiary butyl chloride
9H-carbazole
1,3,6,8-tetra-tert-butyl-9H-carbazole
zinc(II) chloride
4-(3,6-di-tert-butyl-9H-carbazole-9-yl)benzaldehyde
3,6-di-tert-butyl-9-(4-nitro-phenyl)-9H-carbazole
9-(4-bromophenyl)-3,6-bis(1,1-dimethylethyl)-9H-carbazole
3,6-di-tert-butyl-9-(4-iodophenyl)-9H-carbazole
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:1060735-14-9
Molecular Formula:C30H20N2
Molecular Weight:408.5