Your Location:Home >Products >OLED intermediates >Carbazoles >131409-18-2

Product Details
The present invention provides the compound represented by Formula 1, an organic electric element comprising a first electrode, a second electrode, and an organic material layer formed between the first electrode and the second electrode, and electronic d
The present specification relates to a heterocyclic compound represented by chemical formula 1, and an organic light emitting device comprising the same. The life characteristics of the device can be improved by thermal stability of the compound.
In the present invention, provided are a novel compound to improve light efficiency, the stability and the life service of elements, an organic electronic element using the same, and an electronic device thereof. More specifically, the organic electronic element of the present invention includes: a first electrode, a second electrode, an organic material layer located between the first electrode and the second electrode, and an improvement layer formed on one side to improve light efficiency.
The present invention provides a compound of chemical formula 1 and an organic light emitting device comprising the same. The compound according to the present invention has excellent lifespan characteristics and can have high luminous efficiency even at a low driving voltage.COPYRIGHT KIPO 2020
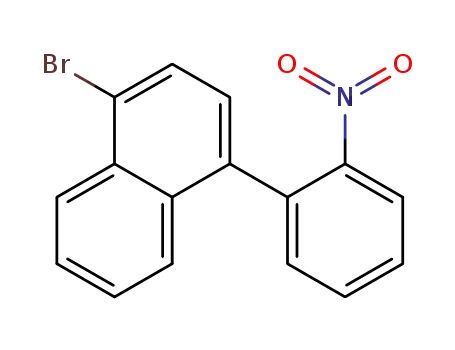
1-bromo-4-(2-nitrophenyl)naphthalene

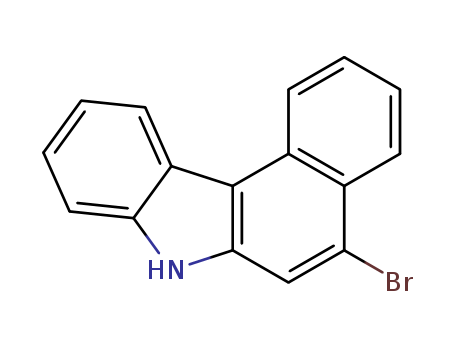
![5-bromo-7H-benzo[c]carbazole](/upload/2023/2/707f345e-d8dd-4659-be84-ca8ab10da112.png)
5-bromo-7H-benzo[c]carbazole
| Conditions | Yield |
|---|---|
|
With
triphenylphosphine;
In
N,N-dimethyl-formamide;
|
87% |
|
With
triphenylphosphine;
In
1,2-dichloro-benzene;
Reflux;
|
79% |
|
With
triphenylphosphine;
In
1,2-dichloro-benzene;
at 200 ℃;
|
70% |
|
With
triphenylphosphine;
In
1,2-dichloro-benzene;
Reflux;
|
70% |
|
With
iron(II) oxalate dihydrate;
at 205 ℃;
for 0.5h;
|
60.5% |
|
With
triethyl phosphite;
In
1,2-dichloro-benzene;
at 140 ℃;
|
52% |
|
With
triethyl phosphite;
In
1,2-dichloro-benzene;
at 150 ℃;
for 24h;
|
52% |
|
With
triphenylphosphine;
In
1,2-dichloro-benzene;
for 5h;
Reflux;
|
|
|
With
triphenylphosphine;
In
1,2-dichloro-benzene;
|
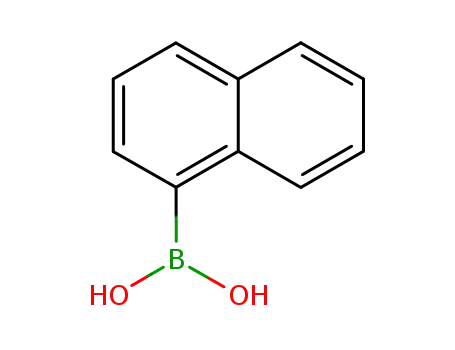
1-Naphthylboronic acid

![5-bromo-7H-benzo[c]carbazole](/upload/2023/2/707f345e-d8dd-4659-be84-ca8ab10da112.png)
5-bromo-7H-benzo[c]carbazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / toluene; ethanol / 3 h / 90 °C
2: iron(III) chloride; bromine / tetrachloromethane / -5 °C
3: triethyl phosphite / 1,2-dichloro-benzene / 140 °C
With
iron(III) chloride; tetrakis(triphenylphosphine) palladium(0); bromine; potassium carbonate; triethyl phosphite;
In
tetrachloromethane; ethanol; 1,2-dichloro-benzene; toluene;
|
|
|
Multi-step reaction with 3 steps
1: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / toluene; ethanol; water / 2 h / 90 °C
2: bromine / tetrachloromethane / 24 h / 20 °C
3: triethyl phosphite / 1,2-dichloro-benzene / 24 h / 150 °C
With
tetrakis(triphenylphosphine) palladium(0); bromine; potassium carbonate; triethyl phosphite;
In
tetrachloromethane; ethanol; water; 1,2-dichloro-benzene; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / tetrahydrofuran; water / 0.25 h / Inert atmosphere
1.2: 5 h / Inert atmosphere; Reflux
2.1: bromine / tetrachloromethane / 24 h / Reflux
3.1: triphenylphosphine / 1,2-dichloro-benzene / 5 h / Reflux
With
bromine; potassium carbonate; triphenylphosphine;
In
tetrahydrofuran; tetrachloromethane; water; 1,2-dichloro-benzene;
|
|
|
Multi-step reaction with 3 steps
1: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / toluene; ethanol / 12 h / Reflux
2: N-Bromosuccinimide / dichloromethane / 12 h / 20 °C
3: iron(II) oxalate dihydrate / 0.5 h / 205 °C
With
N-Bromosuccinimide; tetrakis(triphenylphosphine) palladium(0); iron(II) oxalate dihydrate; potassium carbonate;
In
ethanol; dichloromethane; toluene;
|
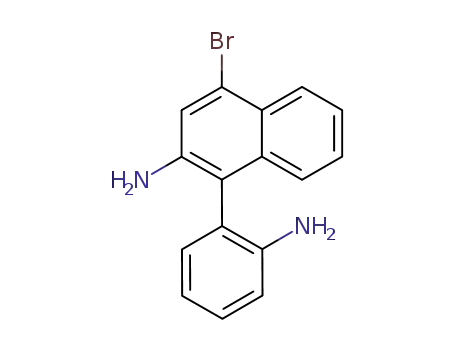
1-(2-amino-phenyl)-4-bromo-[2]naphthylamine

1-Naphthylboronic acid
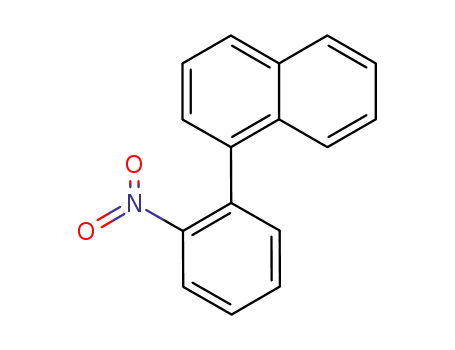
1-(2-nitrophenyl)naphthalene
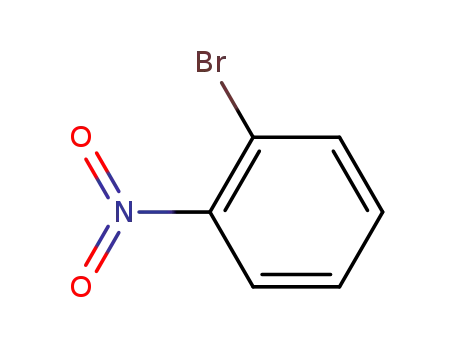
2-nitrophenyl bromide
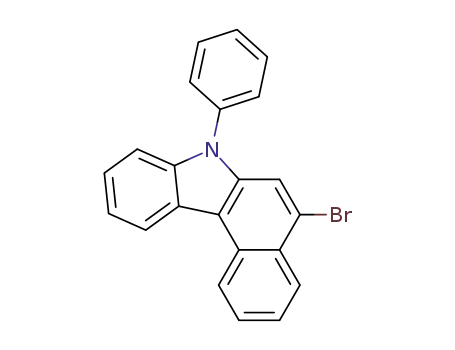
5-bromo-7-phenyl-7H-benzo[c]carbazole
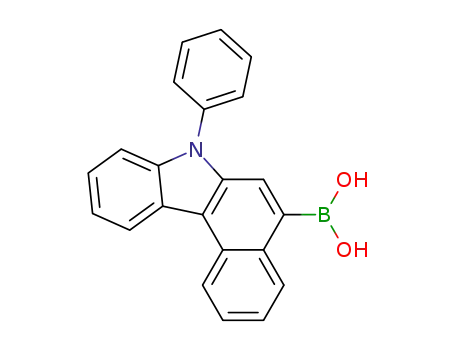
(7-phenyl-7H-benzo[c]carbazol-5-yl)boronic acid
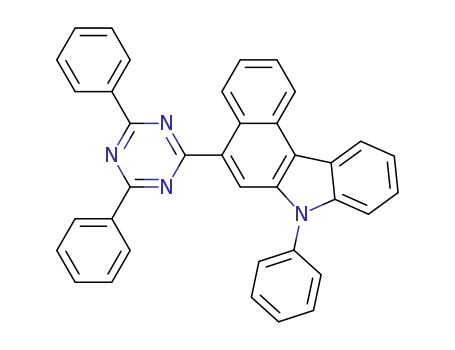
C37H24N4
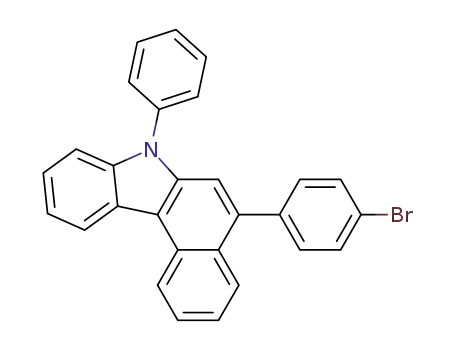
C28H18BrN
CAS:69249-61-2
CAS:1210469-11-6
Molecular Formula:C22H14BrN
Molecular Weight:372.3
CAS:1060735-14-9
Molecular Formula:C30H20N2
Molecular Weight:408.5