Your Location:Home >Products >OLED intermediates >Carbazoles >57103-20-5

Product Details
Chemical Properties
off-white powder
InChI:InChI=1/C18H11Br2N/c19-12-6-8-17-15(10-12)16-11-13(20)7-9-18(16)21(17)14-4-2-1-3-5-14/h1-11H
The invention discloses a preparation method of N-arylcarbazole-3-boric acid, and belongs to the field of liquid crystal intermediates. The preparation method comprises the following steps: coupling carbazole with aryl halide in the presence of alkali to generate N-arylcarbazole, enabling the N-arylcarbazole to react with a bromination reagent to generate Naryl-3, 6-dibromo carbazole, enabling theNaryl-3, 6-dibromo carbazole to react with borate and butyl lithium by a one-pot method, and carrying out hydrolyzing to obtain N-arylcarbazole-3-boric acid. According to the method, dibromides whichare easy to purify are generated during bromination, monosubstituted products are generated by controlling the using amount of the lithiation reagent and the boric acid ester during lithiation, the method is verified on the scale of dozens of kilograms, and the method has the prospect of industrial methods.
The invention belongs to the field of perovskite solar cell materials, and discloses a carbazole-based small organic molecule hole transport material. According to the carbazole-based small organic molecule hole transport material, a carbazole intermediate core group fragment is synthesized by utilizing a low-temperature reaction and a bromination reaction; a target product OY1, a target product OY2 and a target product OY3 are obtained by means of synthesis from the carbazole intermediate core group fragment and peripheral triphenylamine micromolecules through an acylation reaction and a coupling reaction; the structures of the target product OY1, the target product OY2 and the target product OY3 are confirmed through a high-resolution mass spectrum characterization means, and the target product OY1, the target product OY2 and the target product OY3 are applied to a perovskite solar cell; the synthesized compound is subjected to photophysic, electrochemical, intrinsic hole mobility and thin film characteristic tests, and in addition, the photovoltaic performance of the perovskite solar cell is represented through tests of output current-voltage, incident monochromatic photon-electron conversion efficiency, long-term stability and the like of the cell device. Due to low cost, easy chemical synthesis and excellent performance, the carbazole-based small organic molecule hole transport material will become a powerful competitor.
A simple and cost-effective protocol for the C–N cross coupling of indole derivatives with aryl iodides using CuI/phenanthroline catalytic system in aqueous and DME/H2O solvent mixture is described. The reactions were performed in the absence of phase-transfer catalyst, and afforded N-arylated products in moderate to excellent yields under mild reaction conditions. A systematic tuning of reaction conditions using DME as a co-solvent enables to improve product yields of N-arylation reactions. The broad substrate scope, easy performance, and low loading of catalyst as well as ligand render this approach appropriate for large scale processes. The mechanism of “on water” Cu-catalyzed N-arylation reaction is investigated using kinetic and computational studies, which reveal interesting mechanistic aspects of the reaction. A series of kinetic experiments showed significant rate enhancement for “on water” Cu-catalyzed N-arylation over the reaction performed in the organic solvent (DME). Computational studies corroborated “on water” rate acceleration by delineating the role of water in the reaction. The water induces rate acceleration by stabilizing the transition state of oxidative addition through hydrogen bonding interactions, presumably at the oil-water interface, and thus helps to reduce the free energy of activation of oxidative addition of iodobenzene to the Cu complex, which is identified as the rate-limiting step of reaction.
The present invention provides a pyrimidine-based functional group-containing monomolecular compound represented by chemical formula 1 of Ar-(R_1-R_2-Py)_n as a material for an organic layer of an organic electronic device. The compound can form an organic layer within a short time through photo-curing at room temperature. In chemical formula 1, n is 2-10; Ar is a substituted or non-substituted C_6-C_60 aryl group, or a substituted or non-substituted C_3-C_60 heteroaryl group having an n-valent linking group; each of R_1 and R_2 independently represents a single bond, -O-, a substituted or non-substituted C_6-C_30 arylene group, a substituted or non-substituted C_3-C_30 heteroarylene group, a substituted or non-substituted C_1-C_10 alkylene group, a substituted or non-substituted C_1-C10 alkoxylene group, a substituted or non-substituted amide group, or a substituted or non-substituted amine group; and Py is a monovalent linking group derived from a pyrimidine-based functional group.COPYRIGHT KIPO 2020
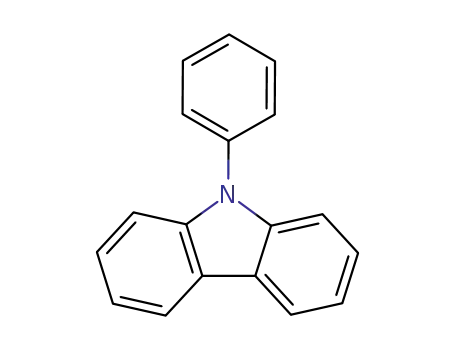
N-phenylcarbazole

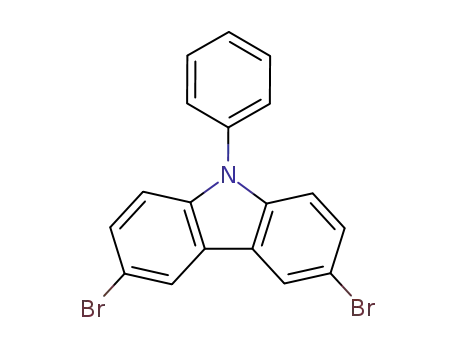
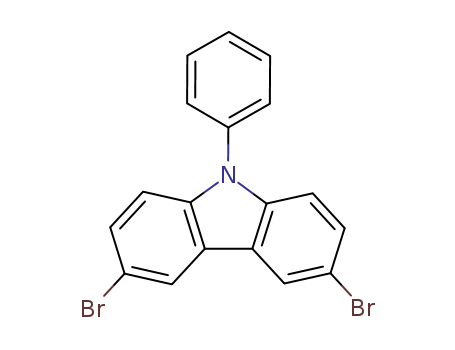
3,6-dibromo-9-phenyl-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
at 20 ℃;
for 12h;
Inert atmosphere;
|
100% |
|
With
bromine; acetic acid;
Ambient temperature;
|
98% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
95% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;
In
methanol;
at 20 ℃;
for 1h;
Solvent;
Temperature;
Reagent/catalyst;
|
95% |
|
With
N-Bromosuccinimide;
In
ethyl acetate;
at 20 ℃;
for 52h;
|
92% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 - 25 ℃;
for 24.5h;
Inert atmosphere;
|
90.3% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 - 25 ℃;
for 24.5h;
Inert atmosphere;
|
90% |
|
With
N-Bromosuccinimide;
In
chloroform;
at 20 ℃;
for 3h;
|
87% |
|
With
bromine; acetic acid;
In
dichloromethane;
at 20 ℃;
Cooling with ice;
|
87.8% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
84% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 3h;
|
80% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 5h;
|
79.04% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
64% |
|
With
bromine;
In
dichloromethane; acetic acid;
|
56.9% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 0 ℃;
for 12h;
Darkness;
|
50% |
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 0 ℃;
Darkness;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
bromine;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 24h;
|
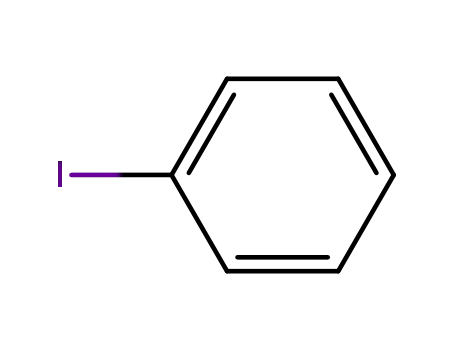
iodobenzene

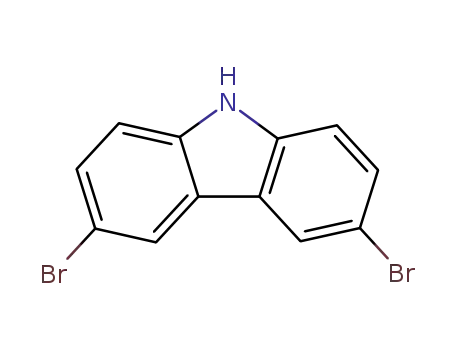
3,6-dibromo-9H-carbazole

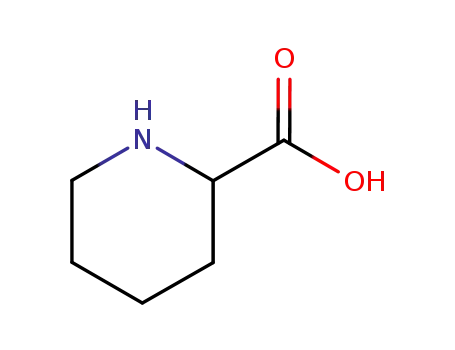
pipecolic Acid


3,6-dibromo-9-phenyl-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
copper(l) iodide; caesium carbonate;
In
N,N-dimethyl-formamide;
at 110 ℃;
for 12h;
Inert atmosphere;
|
90.5% |

N-phenylcarbazole
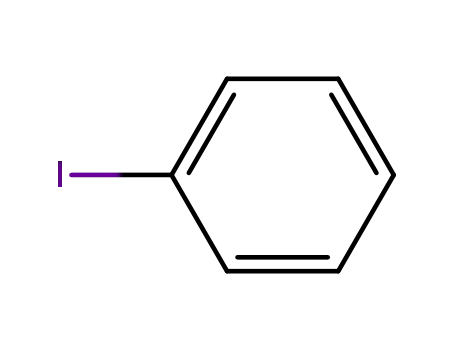
iodobenzene
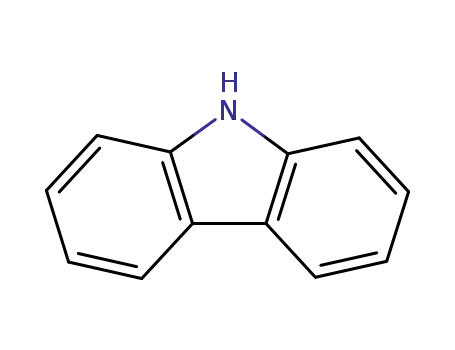
9H-carbazole

3,6-dibromo-9H-carbazole
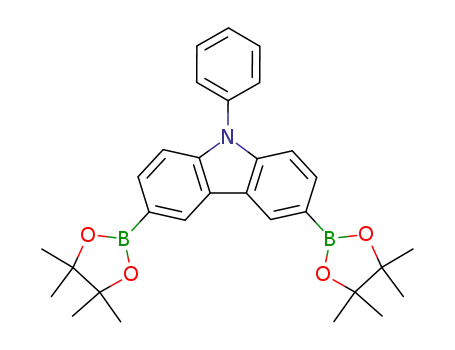
9-phenyl-3,6-bis-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole
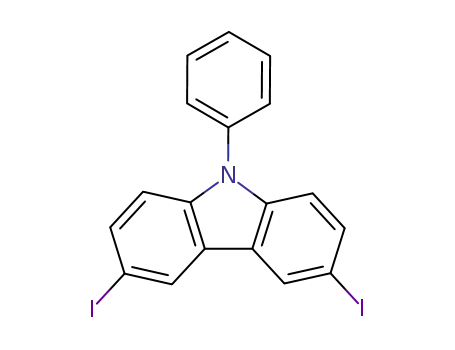
3,6-diiodo-9-phenyl-9H-carbazole
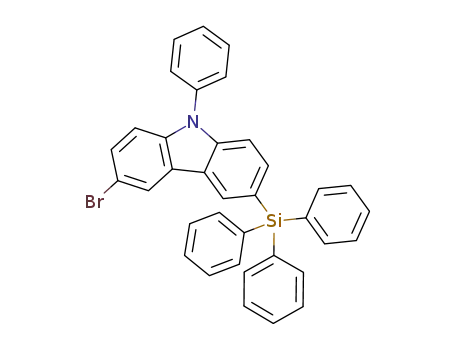
3-bromo-9-phenyl-6-(triphenylsilyl)-9-carbazole
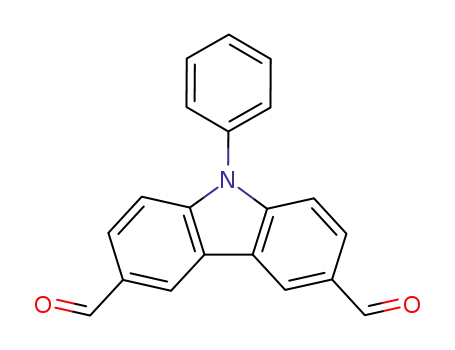
9-phenyl-9H-carbazole-3,6-dicarbaldehyde
CAS:755017-61-9
CAS:1150-62-5
CAS:502161-03-7