Your Location:Home >Products >OLED intermediates >Fluorenes >355832-04-1
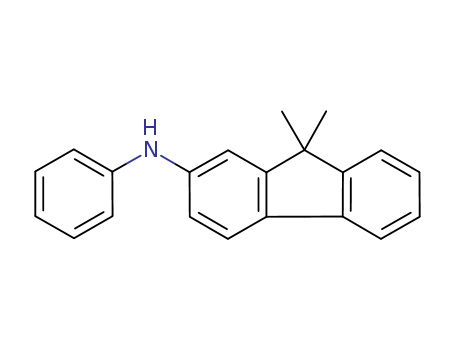

Product Details
Uses
9,9-Dimethyl-N-phenyl-9H-fluoren-2-amine is useful for the synthetic preparation of secondary amines.
To further develop a fluorescent light emitting material so as to obtain a higher element efficiency, a longer lifetime of the device, and more blue emission.SOLUTION: The invention discloses aromatic amine derivative, and an organic electroluminescent device including the same. In a structure, fluorene/silafluorene groups with ortho-substituents are introduced into the aromatic amine derivative, therefore the aromatic amine derivative can be used as light-emitting materials in a light-emitting layer of the organic electroluminescent device. These novel compounds can provide better device performance.SELECTED DRAWING: None
The invention relates to an organic compound, an electronic device comprising the organic compound, and electronic equipment comprising the electronic device. The structural formula of the organic compound is represented by a chemical formula 1, and the organic compound is applied to the electronic device and can significantly improve the performance of the electronic device.
Disclosed are an amine-based compound and an organic light emitting device including the same. The amine-based compound is represented by chemical formula 1A.
The invention provides a nitrogen-containing compound, an organic electroluminescent device and an electronic device, and belongs to the technical field of organic materials. The structure of the nitrogen-containing compound is represented by Chemical Formula 1: wherein X1, X2, Y1, Y2 are the same or different from each other and are each independently a single bond, O, S, N(R3), C(R4R5), Ge(R6R7), Si(R8R9), Se, wherein X1 and Y1 are not single bonds simultaneously and X2 and Y2 are not single bonds simultaneously.
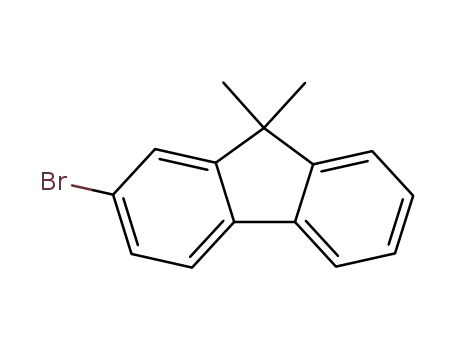
2-bromo-9,9-dimethyl-9H-fluorene

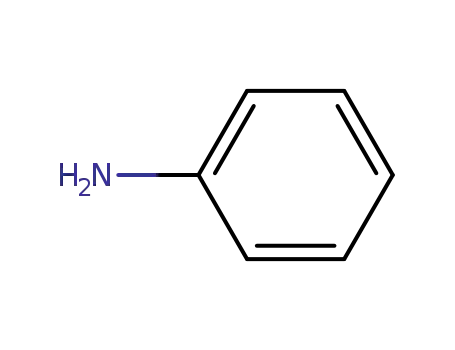
aniline

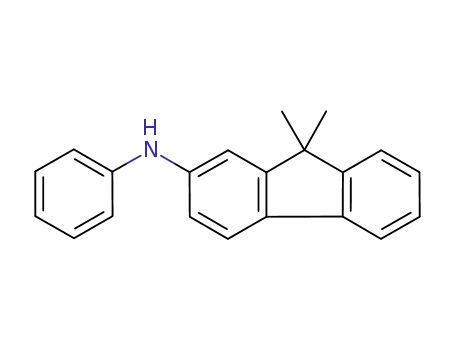
N-phenyl-(9,9-dimethyl-9H-fluoren-2-yl)amine
| Conditions | Yield |
|---|---|
|
With
1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; sodium t-butanolate;
In
toluene;
for 20h;
Reflux;
Inert atmosphere;
|
95% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 110 ℃;
for 3.58333h;
Inert atmosphere;
|
93% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
toluene;
at 90 ℃;
for 3h;
|
92% |
|
2-bromo-9,9-dimethyl-9H-fluorene;
With
tri-tert-butyl phosphine; palladium diacetate;
In
toluene;
for 0.25h;
aniline;
With
sodium t-butanolate;
In
toluene;
at 105 ℃;
|
90% |
|
With
1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; sodium t-butanolate;
In
toluene;
for 18h;
Inert atmosphere;
Heating;
|
90% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 70 ℃;
for 1h;
Inert atmosphere;
|
88.6% |
|
With
tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate;
In
toluene;
for 2h;
Inert atmosphere;
Reflux;
|
87% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; potassium tert-butylate;
In
toluene;
at 85 ℃;
for 2h;
|
84% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
toluene;
at 80 ℃;
for 8h;
|
81% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
toluene;
at 90 ℃;
for 3h;
Inert atmosphere;
|
78% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
toluene;
at 90 ℃;
for 5h;
Inert atmosphere;
|
78% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate;
In
toluene;
for 7h;
Inert atmosphere;
Reflux;
|
77% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate;
In
toluene;
at 105 ℃;
for 24h;
Inert atmosphere;
|
77.4% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 100 - 110 ℃;
for 2h;
Inert atmosphere;
|
77% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate;
In
toluene;
at 105 ℃;
for 24h;
Inert atmosphere;
|
75.5% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
Reflux;
|
75% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
Reflux;
|
75% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
Reflux;
|
75% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
Reflux;
|
74% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
for 4h;
Inert atmosphere;
Reflux;
|
74% |
|
With
1,1'-bis(di-tertbutylphosphino)ferrocene; palladium diacetate; sodium t-butanolate;
In
toluene;
at 90 ℃;
Inert atmosphere;
|
72.1% |
|
With
palladium diacetate; sodium t-butanolate; catacxium A;
In
toluene;
for 6h;
Inert atmosphere;
Reflux;
|
71.6% |
|
With
palladium diacetate; sodium t-butanolate; tri tert-butylphosphoniumtetrafluoroborate;
In
toluene;
for 12h;
Inert atmosphere;
Reflux;
|
67.44% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate;
In
toluene;
at 100 ℃;
for 24h;
|
64% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
toluene;
at 20 ℃;
for 5h;
|
|
|
With
tri-tert-butyl phosphine; sodium t-butanolate;
palladium diacetate;
In
toluene;
at 100 ℃;
for 12h;
|
|
|
With
tri-tert-butyl phosphine; palladium diacetate; caesium carbonate;
In
toluene;
for 12h;
Reflux;
|
|
|
With
tris-(dibenzylideneacetone)dipalladium(0); potassium tert-butylate; triphenylphosphine;
In
toluene;
at 130 ℃;
for 12h;
Inert atmosphere;
|
|
|
With
tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate;
In
toluene;
at 105 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 5h;
Inert atmosphere;
|
|
|
With
dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 5h;
Inert atmosphere;
|
C23H21NO


N-phenyl-(9,9-dimethyl-9H-fluoren-2-yl)amine
| Conditions | Yield |
|---|---|
|
With
triethyl borane; sodium hydroxide;
In
tert-butyl methyl ether;
at 80 ℃;
for 6h;
Inert atmosphere;
Sealed tube;
|
89% |
|
C23H21NO;
With
Triethoxysilane; sodium triethylborohydride;
In
tert-butyl methyl ether;
at 80 ℃;
for 6h;
With
hydrogenchloride;
In
tert-butyl methyl ether; water;
at 20 ℃;
for 1h;
chemoselective reaction;
|
88% |
|
Multi-step reaction with 2 steps
1: potassium hydroxide; triethyl borane / tetrahydrofuran / 24 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube
2: sodium hydroxide; water / tetrahydrofuran / 1 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube
With
triethyl borane; water; potassium hydroxide; sodium hydroxide;
In
tetrahydrofuran;
|
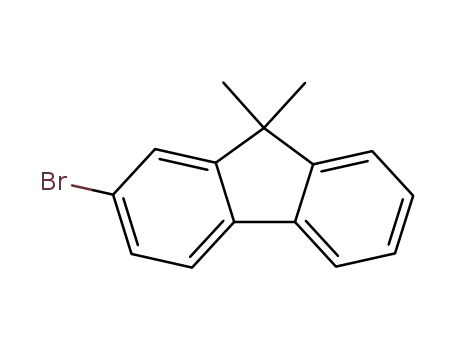
2-bromo-9,9-dimethyl-9H-fluorene

aniline
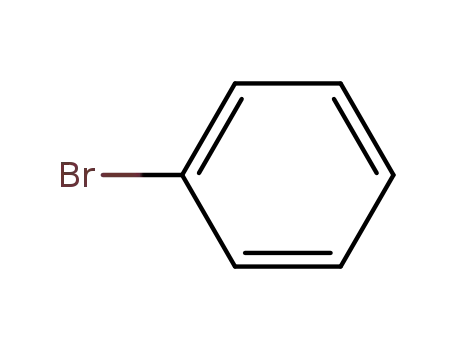
bromobenzene
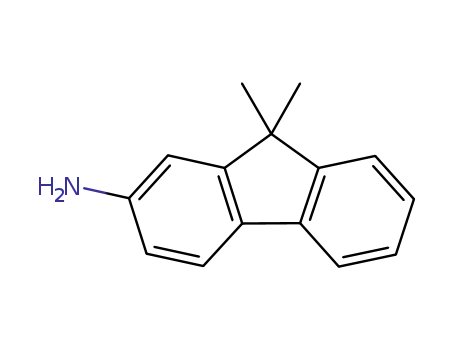
9,9-dimethyl-9H-fluoren-2-ylamine
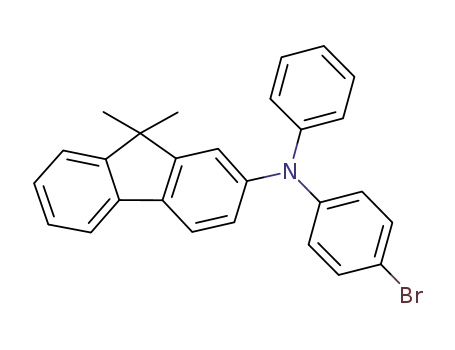
N-phenyl-1-(4-bromophenyl)-(9,9-dimethyl-9H-fluoren-2-yl)amine
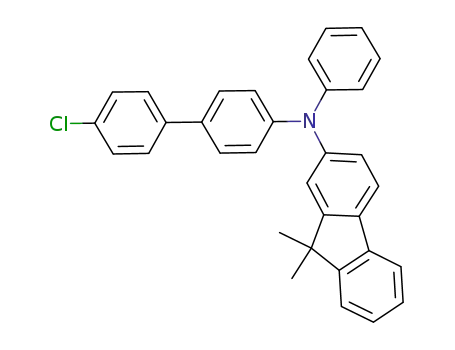
C33H26ClN
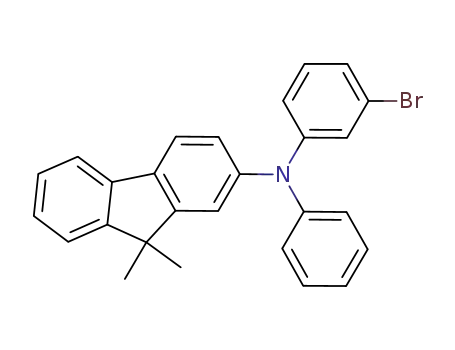
2-((3-bromophenyl)(phenyl)methyl)-9,9-dimethyl-9H-fluorene
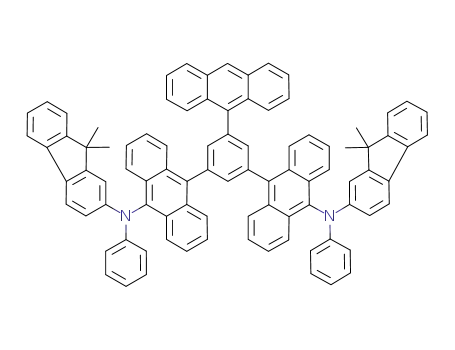
C90H64N2
CAS:97511-05-2
CAS:932710-63-9
Molecular Formula:C16H28NP
Molecular Weight:265.37
CAS:500717-23-7
Molecular Formula:C30H27N
Molecular Weight:401.5