Your Location:Home >Products >Organic phosphines >Phenyl phosphines >2071-20-7
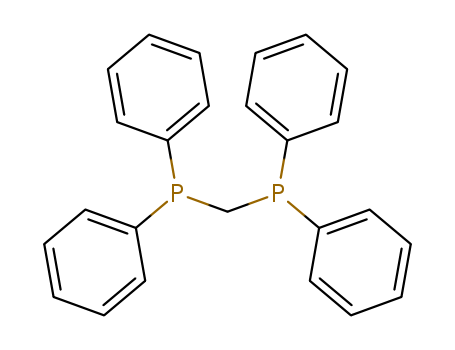

Product Details
Chemical Properties
white to light yellow crystal powde
Uses
suzuki reaction
Uses
It is applied as an organic synthesis catalyst. It is also used as an intermediate in flavor and fragrances.
InChI:InChI=1/C25H22P2/c1-5-13-22(14-6-1)26(23-15-7-2-8-16-23)21-27(24-17-9-3-10-18-24)25-19-11-4-12-20-25/h1-20H,21H2
A titanocene carbene complex reacted with diphosphine monoxide to give R2PCH2PR2.
We report a variety of manganese-based catalysts containing both chelating diphosphine (bis(diphenylphosphino)methane (dppm: 1, 2, and 7) or 1,2-bis(diphenylphosphino)ethane (dppe: 3)), and mixed-donor phosphinoamine (2-(diphenylphosphino)ethylamine (dppea: 4-6)) ligands for the upgrading of ethanol and methanol to the advanced biofuel isobutanol. These catalysts show moderate selectivity up to 74% along with turnover numbers greater than 100 over 90 h, with catalyst 2 supported by dppm demonstrating superior performance. The positive effect of substituting the ligand backbone was also displayed with a catalyst supported by C-phenyl-substituted dppm (8) having markedly improved performance compared to the parent dppm catalysts. Catalysts supported by the phosphinoamine ligand dppea are also active for the upgrading of ethanol to n-butanol. These results show that so-called PNP-pincer ligands are not a prerequisite for the use of manganese catalysts in Guerbet chemistry and that simple chelates can be used effectively.
Photoirradiation on 02(dppm)3> (dppm = bis(diphenylphosphino)methane) in 1,2-dichloroethane solution with visible light yields I2Cl2(dppm)2> (2) and ethylene quantitatively.Irradiation of 2 in various solutions shows interesting photochromic behavior.
The attempted synthesis of bis(diphenylphosphinomethyl) phenylarsane and tris(diphenylphosphinomethyl) arsane through condensation of chloro arsanes and diphenyl (trimethylsilylmethyl) phosphane yielded a number of side products originating from migratory and redox-reactions in addition to the targeted ligands. An unexpected, 1,3,4-phosphadiarssolan-1-ium salt was obtained and crystallographically characterized as an A-shaped chlorido adduct.
Disclosed herein is a catalyst system for selective oligomerization of ethylene, which comprises a P—C—C—P frame-work ligand, which is (R1)(R2)P—(R5)CHCH(R6)—P(R3)(R4), and a chromium-based metal compound. Also disclosed is a method of greatly enhancing the activity and selectivity of oligomerization, such as trimerization or tetramerization, using a ligand having a specific steric arrangement structure.
Disclosed herein is a method of preparing 1-octene at high activity and high selectivity while stably maintaining reaction activity by tetramerizing ethylene using a chromium-based catalyst system comprising a transition metal or a transition metal precursor, a cocatalyst, and a P—C—C—P backbone structure ligand represented by (R1)(R2)P—(R5)CHCH(R6)—P(R3)(R4).
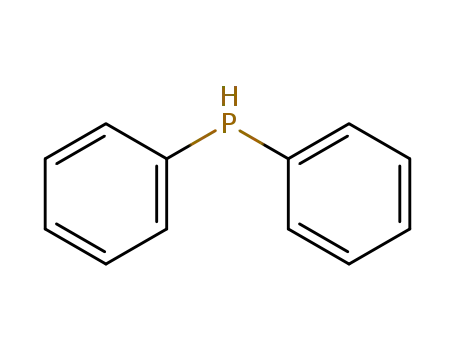
diphenylphosphane

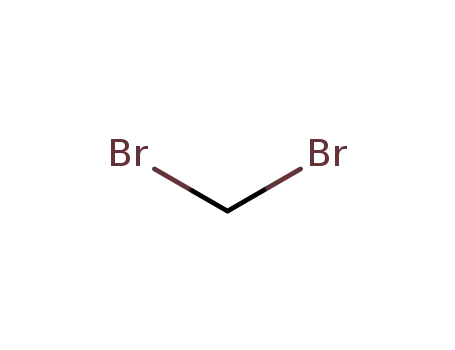
1,1-dibromomethane

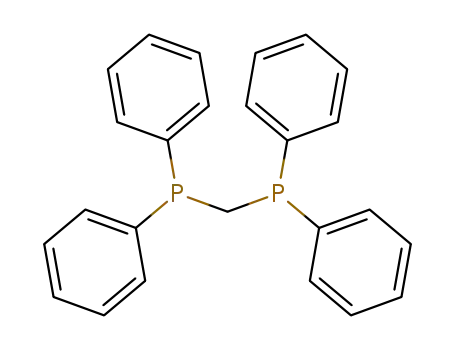
bis-diphenylphosphinomethane
| Conditions | Yield |
|---|---|
|
With
cesium hydroxide; 4 Angstroem MS;
In
N,N-dimethyl-formamide;
at 23 ℃;
for 16h;
|
95% |
|
With
cesium hydroxide; 4 A molecular sieve;
In
N,N-dimethyl-formamide;
at 23 ℃;
for 16h;
|
95% |
|
diphenylphosphane;
With
cesiumhydroxide monohydrate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
Molecular sieve;
Inert atmosphere;
1,1-dibromomethane;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 16h;
Inert atmosphere;
Molecular sieve;
|
95% |
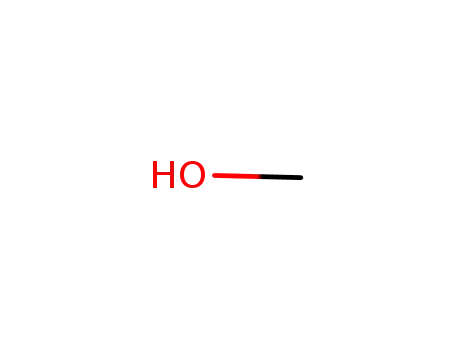
methanol

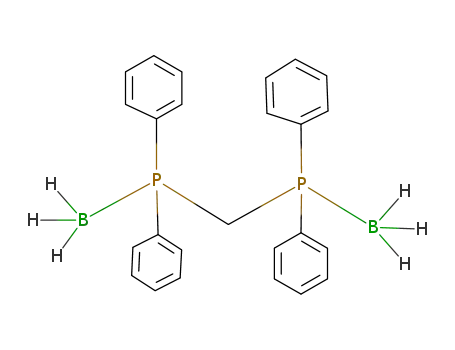
methylenebis(diphenylphosphane)-borane(1:2)

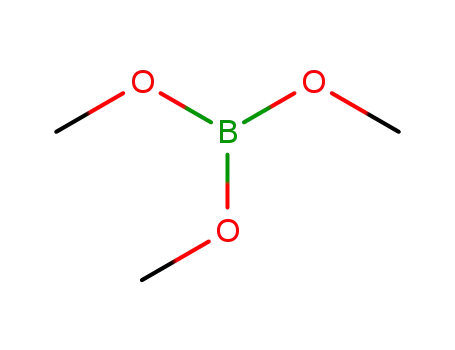
Trimethyl borate


bis-diphenylphosphinomethane
| Conditions | Yield |
|---|---|
|
In
toluene;
at 100 ℃;
Inert atmosphere;
|
100% |
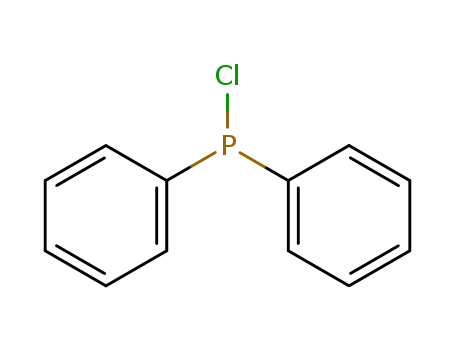
chloro-diphenylphosphine
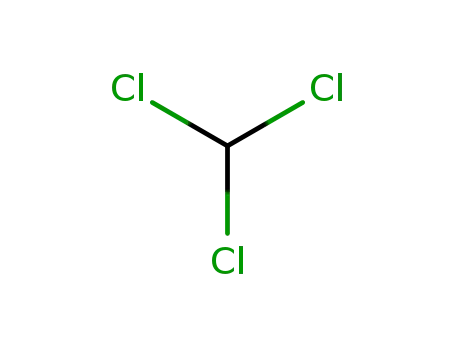
chloroform
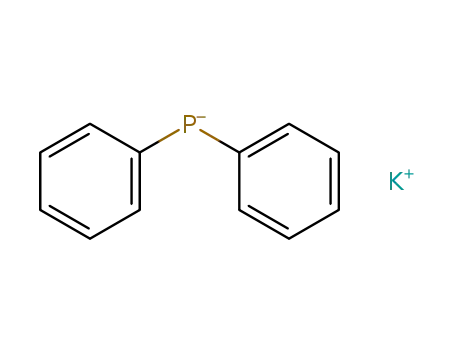
potassium diphenylphosphine
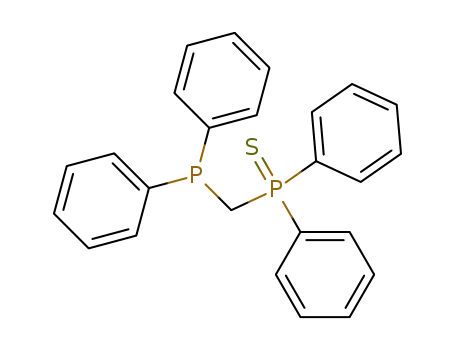
(diphenylphosphinomethyl)diphenylphosphine sulphide
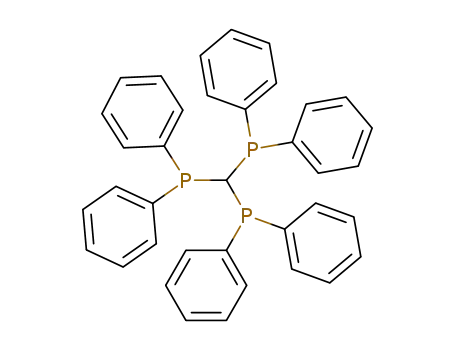
Tris(diphenylphosphino)methan
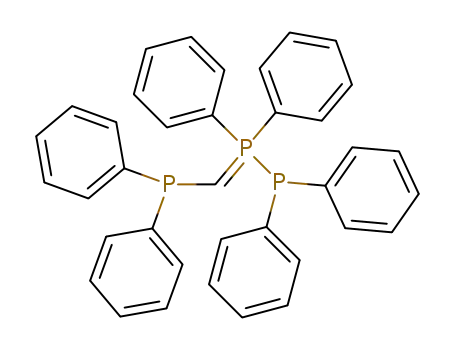
C37H31P3
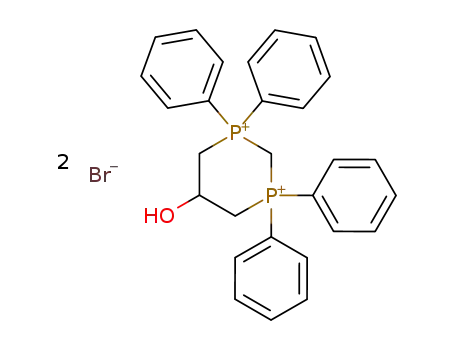
5-hydroxy-1,1,3,3-tetraphenyl-[1,3]diphosphinanediium; dibromide
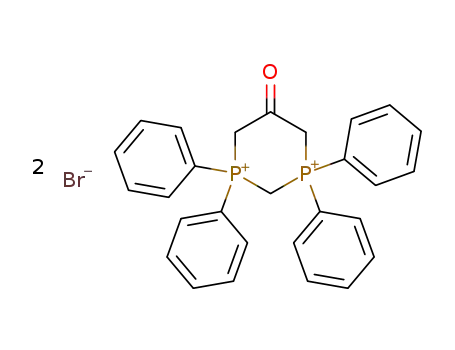
5-oxo-1,1,3,3-tetraphenyl-[1,3]diphosphinanediium; dibromide
CAS:502161-03-7
CAS:1428551-28-3
Molecular Formula:C24H16BrN
Molecular Weight:398.3
CAS:13885-09-1