Your Location:Home >Products >Norbornenes >95-10-3
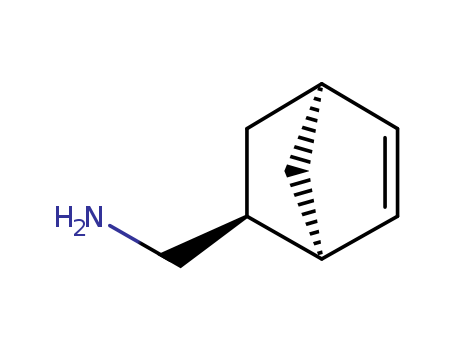
Product Details
InChI:InChI=1/C8H13N/c9-5-8-4-6-1-2-7(8)3-6/h1-2,6-8H,3-5,9H2
A two-catalyst system comprising of salen(AlCl) and pybox(ErCl3) complexes supported onto poly(norbornene) has been developed. As a proof of concept the activity of the two-catalyst system was gauged for the addition of cyanide to α,β-unsaturated imides which has been shown to follow a bimetallic mechanism. The activity of the supported two-catalyst system was significantly higher than catalytic systems derived from mixtures of the two catalysts. In addition, the co-polymer could be readily recovered and reused up to 3 cycles without significant loss in conversions and yields. Nevertheless, a decrease in enantioselectivity of the cyanide adduct was observed with each subsequent cycle indicating some loss in catalytic selectivity. The reported strategy opens up avenues for supporting multiple catalysts on the same polymer backbone for catalyst-based one-pot cascade reactions.
Embodiments in accordance with the present invention are directed to providing processes for forming fluorinated-alkyl and particularly perfluorinated-alkyl sulfonamide substituted norbornene-type monomers.
Electron density distribution in molecules of amino derivatives of norbornene, exo- and endo-5-aminomethylbicyclohept-2-ene, exo-5-aminomethyl-endo-5-methylbicyclohept-2-ene, and their epoxy derivatives was studied by spectral methods (IR and 1H, 13C, and two-dimensional NMR spectroscopy) and by semiempirical (AM1) quantum-chemical calculations.The reactivity of amines was preliminary estimated from the calculated parameters: proton affinity and energies of localized molecular orbitals.The nucleophilic properties of the amines were studied in reactions with acyclic, alicyclic, and aromatic isocyanates and isothiocyanates.In the series of potentially bioactive ureas and thioureas, criteria have been formulated for ascribing the exo and endo structures to these compounds on the basis of the 1H NMR spectra.
Fluorinated sulfonamides derived from exo- and endo-5-aminomethylbicyclohept-2-ene have been prepared and studied.These compounds were synthesized from pure isomers of bicyclohept-2-ene-5-carbonitrile obtained by Diels-Alder reaction from cyclopentadiene and acrylonitrile.The influence of the substituent orientation in the norbornene moiety on the 1H NMR spectra of stereoisomeric sulfonamides has been studied.Reactions of both isomers of N-(perfluorobutylsulfonyl)-5-aminomethylbicyclohept-2-ene with peroxy acids yield epoxides as the only reaction products.The intramolecular cyclization product is not formed, probably owing to the low nucleophilicity (basicity) of the nitrogen atom bearing the perfluorosulfonyl group.
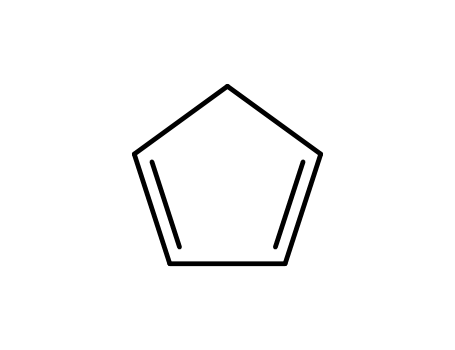
cyclopenta-1,3-diene

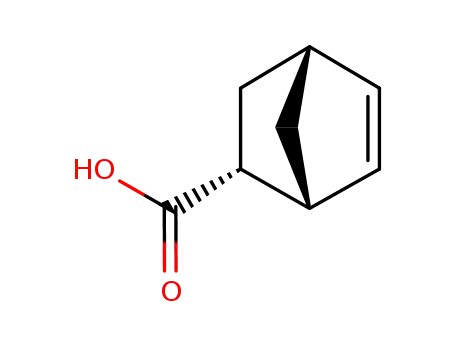
endo-norbornenecarboxylic acid

![endo-5-aminomethylbicyclo[2.2.1]hept-2-ene](/upload/2023/2/d0867092-9601-4b46-91f8-6cf4dbf6f4aa.png)
endo-5-aminomethylbicyclo[2.2.1]hept-2-ene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 1.) triethylamine, ethylchloroformate / 1.) THF, 0 deg C, 10 min; 2.) THF, at 0 deg C, 1 h, at RT, 10 h
2: 1.) cc. HCl, methanol; 2.) 10 percent aqueous KOH / 1.) reflux, 6 h; 2.) reflux, 1 h
With
hydrogenchloride; methanol; potassium hydroxide; chloroformic acid ethyl ester; triethylamine;
|

cyclopenta-1,3-diene

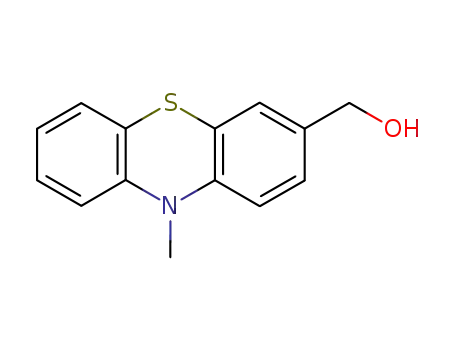
3-hydroxymethyl-10-methyl-10H-phenothiazine

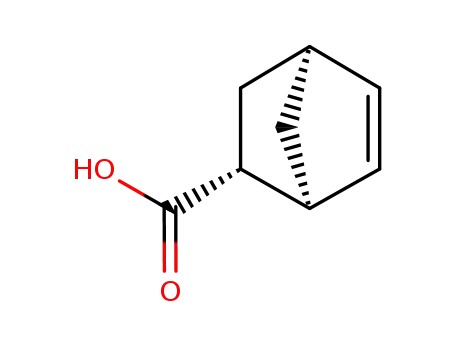
exo-5-norbornene-2-carboxylic acid

C40H30N2O3

C30H26N2O3


exo-5-norbornene-2-carboxylic acid

C22H21NO2S

C48H38N2O4

![2-aminomethylbicyclo[2.2.1]hept-5-ene](/upload/2023/2/0682c354-513d-4a9d-9f5b-e7ff01bd4cff.png)
2-aminomethylbicyclo[2.2.1]hept-5-ene
| Conditions | Yield |
|---|---|
|
With
dmap; dicyclohexyl-carbodiimide;
In
dichloromethane;
for 16h;
Inert atmosphere;
Reflux;
|
81% |
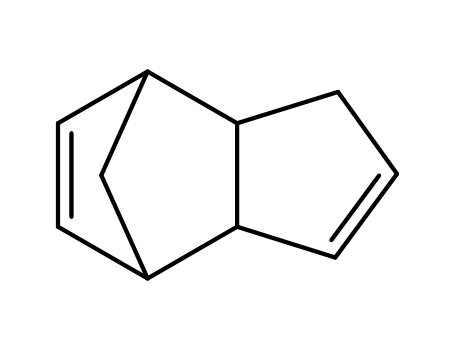
bi(cyclopentadiene)
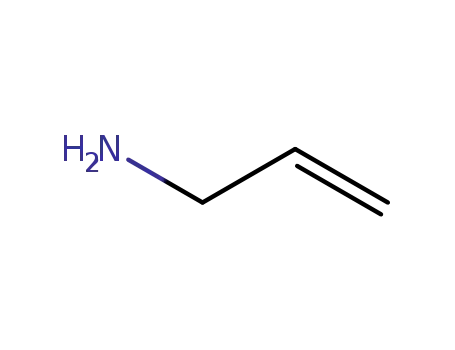
1-amino-2-propene

cyclopenta-1,3-diene

3-hydroxymethyl-10-methyl-10H-phenothiazine
CAS:884336-44-1
CAS:728039-63-2
CAS:159326-68-8
Molecular Formula:C6H6N4
Molecular Weight:134.14