Your Location:Home >Products >Functional intermediates >159326-68-8

Product Details
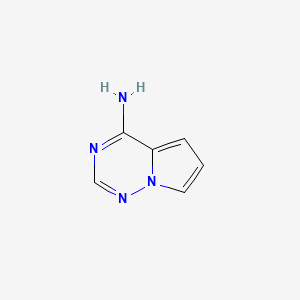
| Isomeric SMILES | C1=CN2C(=C1)C(=NC=N2)N |
| XLogP3-AA | 0.1 |
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 31, p. 781, 1994 DOI: 10.1002/jhet.5570310415 |
Pyrrolo[2,1-f][1,2,4]triazine, a unique bicyclic heterocycle, containing N–N bond with a bridgehead nitrogen, possesses numerous activities against diverse therapeutic targets. It was first synthesized in late 1970s, but did not find many applications thereafter. Pyrrolo[2,1-f][1,2,4]triazine is an important regulatory starting material in the production of the antiviral drug remdesivir. A broad-spectrum antiviral drug remdesivir has been recently recognized against wide array of RNA viruses (including SARS/MERS-CoV) and has shown encouraging results in the treatment of recently emerged novel coronavirus (COVID-19). Also, pyrrolo[2,1-f][1,2,4]triazine is an active structural motif of other drugs such as brivanib alaninate, BMS-690514, and BMS-599626 (EGFR inhibitor in clinical phase II) and many others.
InChI:InChI=1/C6H6N4/c7-6-5-2-1-3-10(5)9-4-8-6/h1-4H,(H2,7,8,9)
In contrast, the substitution at C-2 and C-7 positions of pyrrolo[2,1-f][1,2,4]triazine plays an important role to develop ALK selective inhibitors (Figs. 9, 10, 23). In general, large groups …
Pyrrolo[2,1-f][1,2,4]triazine (1) is an important regulatory starting material in the production of the antiviral drug remdesivir. Compound 1 was produced through a newly developed synthetic methodology utilizing simple building blocks such as pyrrole, chloramine, and formamidine acetate by examining the mechanistic pathway for the process optimization exercise. Triazine 1 was obtained in 55% overall yield in a two-vessel-operated process. This work describes the safety of the process, impurity profiles and control, and efforts toward the scale-up of triazine for the preparation of kilogram quantity.
formamidine acetic acid

N-amino-1H-pyrrole-2-carbonitrile

pyrrolo[2,1-f][1,2,4]triazin-4-amine
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In ethanol; at 65 - 70 ℃; Large scale;
|
83% |
|
With potassium phosphate; In ethanol; for 24h; Reflux; Large scale;
|
71.4% |
|
With potassium carbonate; In ethanol; for 2.5h; Heating;
|
66% |
|
In tert-butyl methyl ether; mineral oil; at 90 - 95 ℃; for 16h;
|
60% |
|
With sodium carbonate; In ethanol; at 78 ℃; for 2.5h;
|
60% |
|
With potassium carbonate; In ethanol; at 80 ℃; for 2h;
|
Ca. 45 g |
|
With potassium phosphate; In ethanol; for 10h; Reflux; Inert atmosphere;
|
1-amino-1H-pyrrole-2-carbonitrile hydrochloride

formamidine acetic acid

pyrrolo[2,1-f][1,2,4]triazin-4-amine
| Conditions | Yield |
|---|---|
|
With potassium carbonate; at 78 ℃; for 15h; Inert atmosphere; Large scale;
|
91% |
|
With potassium phosphate; In ethanol; at 78 ℃; for 18h;
|
81% |
|
With potassium phosphate; In ethanol; at 78 ℃; for 18h;
|
81% |
formamidine acetic acid
N-amino-1H-pyrrole-2-carbonitrile
1-formamidinopyrrole-2-carbonitrile
2-pyrrole aldehyde
5-bromopyrrolo[2,1-f][1,2,4]triazin-4-amine
7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylamine
rac-methyl 4-{4-amino-7-[cyclopropyl(hydroxy)methyl]pyrrolo[2,1-f][1,2,4]triazin-5-yl}-2-methoxybenzoate
rac-{4-amino-5-[3-ethyl-5-fluoro-4-(hydroxymethyl)phenyl]pyrrolo[2,1-f][1,2,4]triazin-7-yl}(cyclopropyl)methanol
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:1770840-43-1
Molecular Formula:C6H5IN4
Molecular Weight:260.04
CAS:95-10-3