Your Location:Home >Products >Functional intermediates >400607-16-1
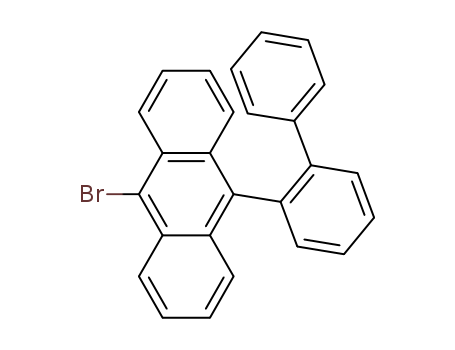
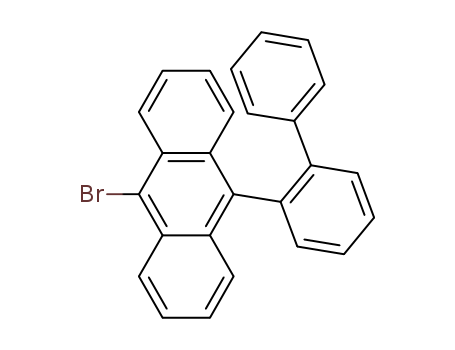
Product Details
Chemical Properties
yellow powder
The invention provides a compound as shown in a general formula (I), which has a mother structure of sym-triphenyl substituted anthracene, is high in bond energy among atoms, has good thermal stability, is beneficial to solid-state accumulation among molecules, is large in bandwidth, has a light-emitting region in a blue light region, is high in light-emitting intensity, and has a proper energy level with adjacent levels. And injection and migration of excitons are facilitated. When the compound is used as a blue light host material in a luminescent layer, the driving voltage of an organic electroluminescent device can be effectively reduced, the luminous efficiency is improved, and the service life is prolonged. The invention also provides an organic electroluminescent device and a display device containing the compound of the general formula (I).
The present invention provides a novel compound and an organic light emitting device using the same. The present invention provides a compound represented by chemical formula 1. The compound represented by chemical formula 1 described above can be used as a material for an organic layer of the organic light emitting device, and can improve efficiency, low driving voltage, and/or lifespan characteristics in the organic light emitting device.
The invention belongs to the technical field of organic materials, and particularly relates to an organic compound and an electronic element and an electronic device using the same, wherein the organic compound has the structure shown 1. When the compound is used as an electron transport layer for preparing an organic electroluminescent device, the service life of the organic electroluminescent device can be effectively prolonged, and the luminous efficiency or the driving voltage can be improved to a certain extent. (by machine translation)
The invention belongs to the field of application sciences of organic photoelectric materials and specifically relates to a naphthyl benzofuran and anthracene derivative, a preparation method and application thereof and a device. The naphthyl benzofuran a
![9‐([1,1'‐biphenyl]‐2‐yl)anthracene](/upload/2023/2/85527364-5bf5-4329-a686-b27afbff7bb1.png)
9‐([1,1'‐biphenyl]‐2‐yl)anthracene

![9-([1,1′-biphenyl]-2-yl)-10-bromoanthracene](/upload/2023/2/346686e7-904d-4c2f-a102-a83432da7001.png)
9-([1,1′-biphenyl]-2-yl)-10-bromoanthracene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 40 ℃;
for 12h;
Inert atmosphere;
|
90% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 2h;
Darkness;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 0 ℃;
for 4h;
|
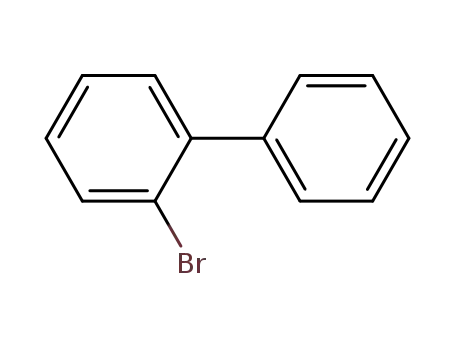
2-Bromobiphenyl

![9-([1,1′-biphenyl]-2-yl)-10-bromoanthracene](/upload/2023/2/346686e7-904d-4c2f-a102-a83432da7001.png)
9-([1,1′-biphenyl]-2-yl)-10-bromoanthracene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: n-butyllithium / hexane; tetrahydrofuran / -78 °C
1.2: -78 - 20 °C
2.1: sodium carbonate / tetrakis(triphenylphosphine) palladium(0) / toluene; water / 3 h / 100 °C
3.1: N-Bromosuccinimide / dichloromethane / 2 h / 20 °C / Darkness
With
N-Bromosuccinimide; n-butyllithium; sodium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran; hexane; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 2 steps
1: potassium carbonate; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran / 12 h / Inert atmosphere; Sonication
2: N-Bromosuccinimide / chloroform; N,N-dimethyl-formamide / 12 h / 40 °C / Inert atmosphere
With
N-Bromosuccinimide; tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; chloroform; N,N-dimethyl-formamide;
|
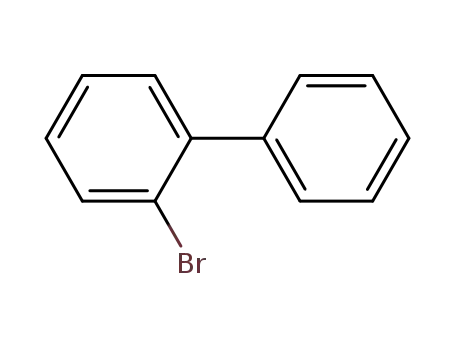
2-Bromobiphenyl
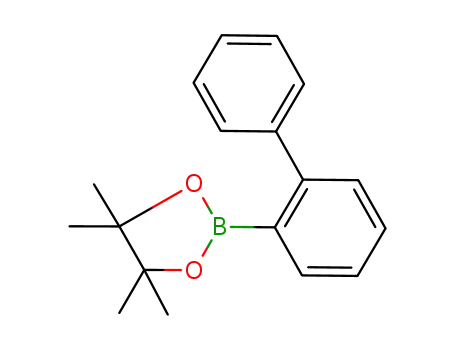
2‐{[1,1'‐biphenyl]‐2‐yl}‐4,4,5,5‐tetramethyl‐1,3,2‐dioxaborolane
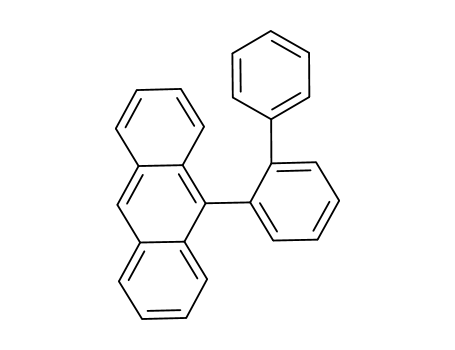
9‐([1,1'‐biphenyl]‐2‐yl)anthracene
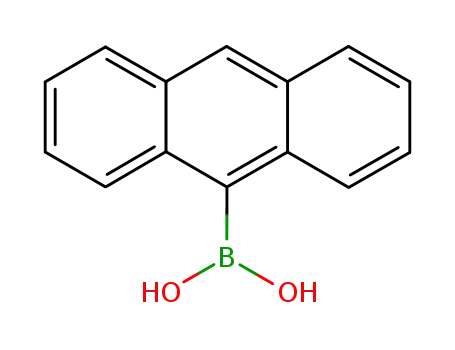
anthracene-9-boronic acid
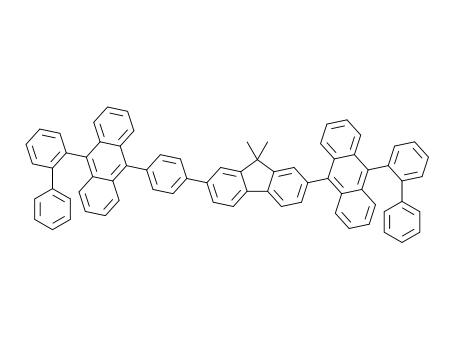
C73H50
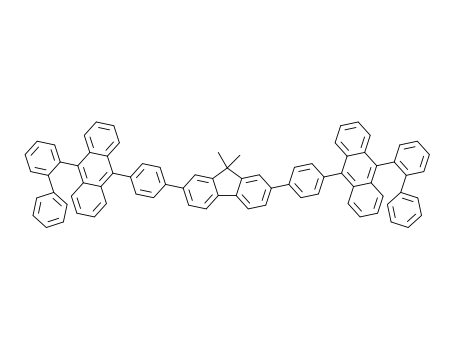
C79H54
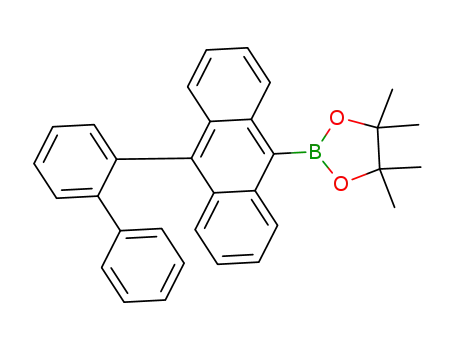
C32H29BO2
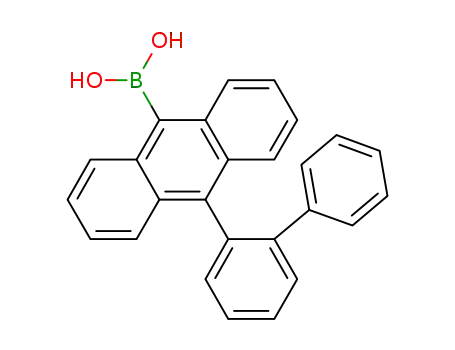
10-([1,1'-biphenyl]-2-yl)-9-anthracenylboronic acid
CAS:1257220-52-2
CAS:1228468-73-2
CAS:2489-86-3