Your Location:Home >Products >Functional intermediates >496-15-1
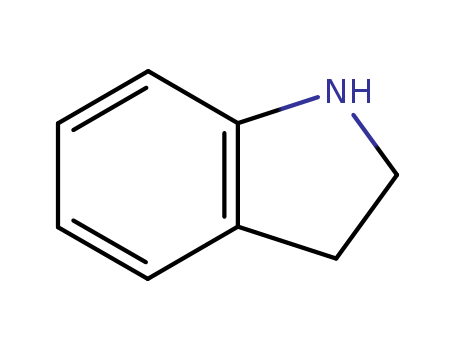

Product Details
Chemical Properties
Clear colorless to slightly brown liquid
Uses
As an indole derivative, Indoline can be used in the preparation of various medicinal compounds such as potential α1-adrenoceptor (α1-AR) antagonists.
Uses
Indole is used in perfumery and in preparing tryptophan, one of the 20 amino acids commonly found in animal proteins. It has important application in the industry of plant growth. It is used to prepare indoleacetic acid (auxin) and other plant growth substances which help the development of roots in plant. Indole and its derivatives are widely used in making perfumes, dyes, agrochemicals and medicines.
Uses
Reactant for preparation of:Inhibitors of NOD1-Induced Nuclear Factor-κB ActivationSphingosine-1-phosphate 4(S1P4) receptor antagonistsCytotoxic cell cycle inhibitors2-AminopyridinesPET agent for imaging of protein kinase C (PKC)Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetesα4β2-Nicotinic acetylcholine receptor-selective partial agonistsmGlu4 positive allosteric modulatorsBacterial biofilm inhibitorsSerotonin 5-HT6 receptor antagonists
Synthesis Reference(s)
Journal of the American Chemical Society, 96, p. 7812, 1974 DOI: 10.1021/ja00832a035Synthetic Communications, 13, p. 489, 1983 DOI: 10.1080/00397918308081827
InChI:InChI=1/C8H9N/c1-2-4-8-7(3-1)5-6-9-8/h1-4,9H,5-6H2
The invention belongs to the technical field of medicines, and discloses an aromatic compound hydrogenation and hydrodeoxygenation method under mild conditions and application of the method in hydrogenation and hydrodeoxygenation reactions of the aromatic compounds and related mixtures. Specifically, the method comprises the following steps: contacting the aromatic compound or a mixture containing the aromatic compound with a catalyst and hydrogen with proper pressure in a solvent under a proper temperature condition, and reacting the hydrogen, the solvent and the aromatic compound under the action of the catalyst to obtain a corresponding hydrogenation product or/and a hydrodeoxygenation product without an oxygen-containing substituent group. The invention also discloses specific implementation conditions of the method and an aromatic compound structure type applicable to the method. The hydrogenation and hydrodeoxygenation reaction method used in the invention has the advantages of mild reaction conditions, high hydrodeoxygenation efficiency, wide substrate applicability, convenient post-treatment, and good laboratory and industrial application prospects.
Developing mild and efficient catalytic methods for the selective synthesis of amines is a longstanding research objective. In this respect, catalytic deoxygenative amide reduction has proven to be promising but challenging, as this approach necessitates selective C–O bond cleavage. Herein, we report the selective hydroboration of primary, secondary, and tertiary amides at room temperature catalyzed by an earth-abundant-metal catalyst, Zr-H, for accessing diverse amines. Various readily reducible functional groups, such as esters, alkynes, and alkenes, were well tolerated. Furthermore, the methodology was extended to the synthesis of bio- and drug-derived amines. Detailed mechanistic studies revealed a reaction pathway entailing aldehyde and amido complex formation via an unusual C–N bond cleavage-reformation process, followed by C–O bond cleavage.
The retinoic acid receptor-related orphan receptor γt (RORγt) is an important nuclear receptor that regulates the differentiation of Th17 cells and production of interleukin 17(IL-17). RORγt agonists increase basal activity of RORγt and could provide a potential approach to cancer immunotherapy. Herein, hit compound 1 was identified as a weak RORγt agonist during in-house library screening. Changes in LHS core of 1 led to the identification of tetrahydroquinoline compound 6 as a partial RORγt agonist (max. act. = 39.3%). Detailed structure-activity relationship on substituent of the LHS core, amide linker and RHS arylsulfonyl moiety was explored and a novel series of tetrahydroquinolines and benzomorpholines was discovered as potent RORγt agonists. Tetrahydroquinoline compound 8g (EC50 = 8.9 ± 0.4 nM, max. act. = 104.5%) and benzomorpholine compound 9g (EC50 = 7.5 ± 0.6 nM, max. act. = 105.8%) were representative compounds with high RORγt agonistic activity in dual FRET assay, and they showed good activity in cell-based Gal4 reporter gene assay and Th17 cell differentiation assay (104.5% activation at 300 nM of 8g; 59.4% activation at 300 nM of 9g). The binding modes of 8g and 9g as well as the two RORγt inverse agonists accidentally discovered were also discussed.
Under the guidance of the known mechanism of the hydrogenation of indoles and transfer hydrogenation with tetrahydroxydiboron (B2(OH)4), Pd/C catalyzed transfer hydrogenation ofN-H indoles with trifluoroethanol and tetrahydroxydiborane as the hydrogen source has been developed. This provides an efficient strategy and catalytic system for the reduction of un-activatedN-H indoles, andN-H indolines are obtained with good to excellent yields. In addition, a series of the isotopic labelling experiments were carried out to probe the mechanism.
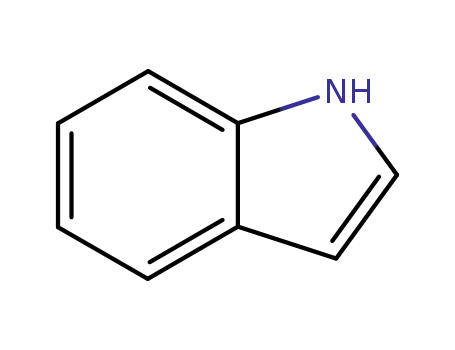
indole

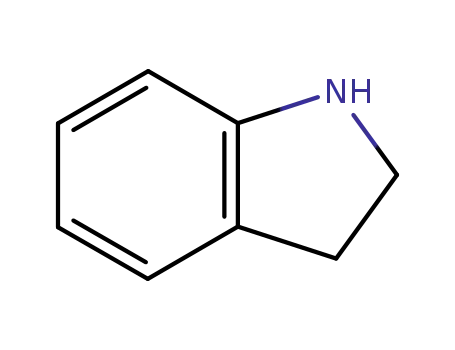
1-indoline

ortho-ethylaniline

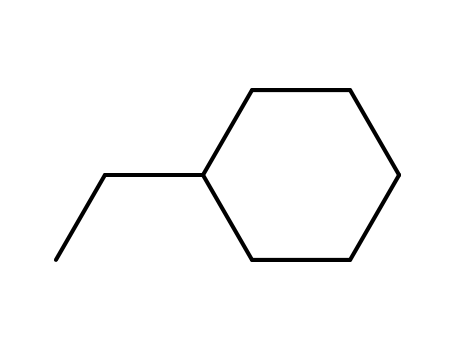
ethyl-cyclohexane

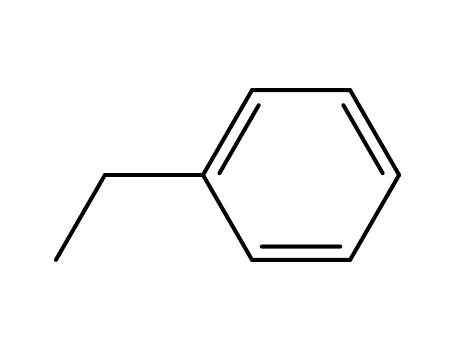
ethylbenzene
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
dodecane;
at 250 ℃;
for 6h;
under 11400.8 Torr;
Reagent/catalyst;
Autoclave;
|
|
|
With
hydrogen;
In
dodecane;
at 250 ℃;
for 6h;
under 11400.8 Torr;
Reagent/catalyst;
Autoclave;
|
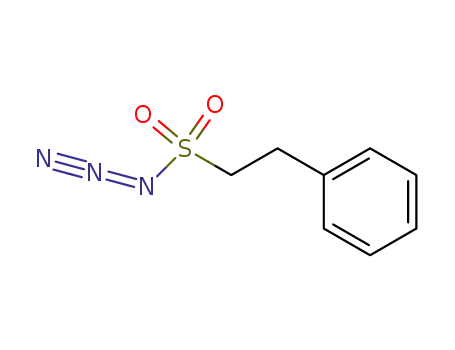
β-phenethylsulfonyl azide

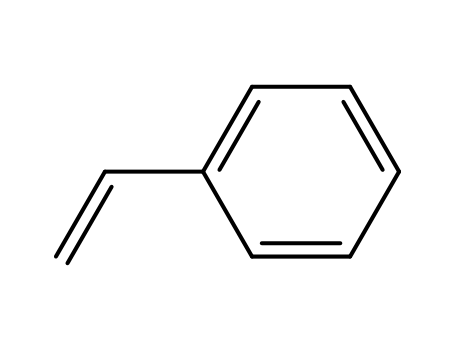
styrene


indole


1-indoline

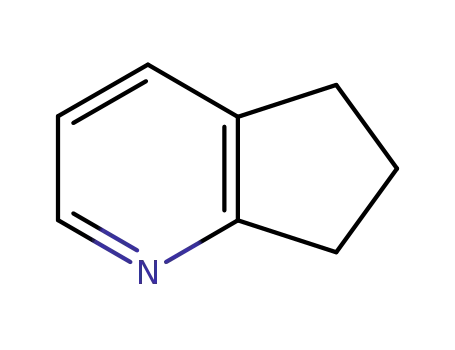
2,3-cyclopentenopyridine
| Conditions | Yield |
|---|---|
|
at 650 ℃;
for 6.94444E-05h;
Product distribution;
other temperature, time;
|
64.8% 11.2% 1.5% 8.2% |

indole
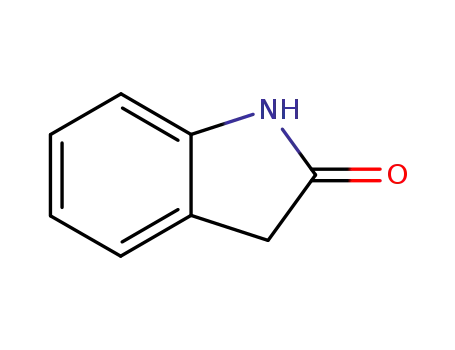
2-oxoindole
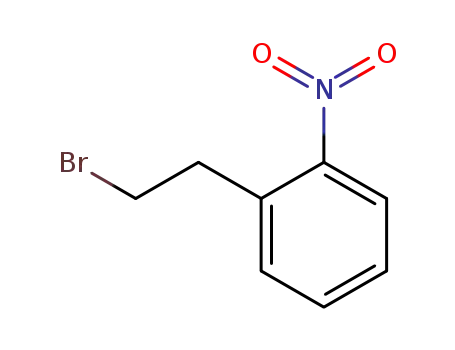
(2-bromoethyl)-2-nitrobenzene
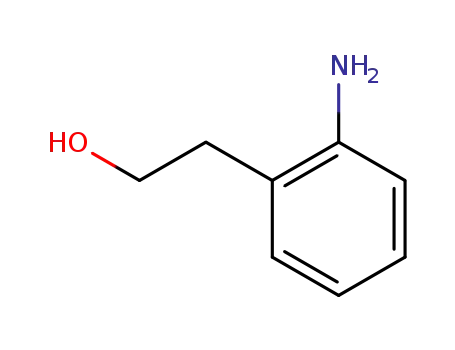
2-aminophenethyl alcohol
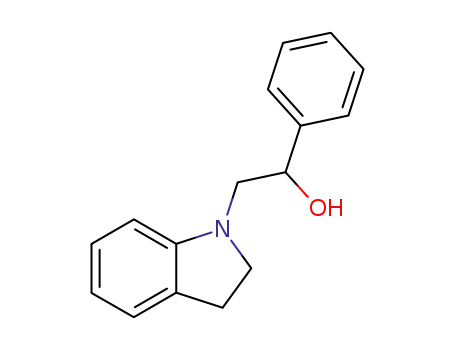
2-indolin-1-yl-1-phenyl-ethanol
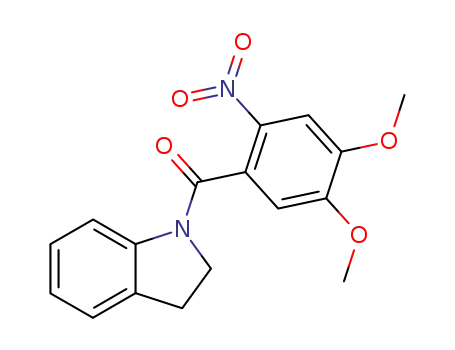
1-(2-nitro-4,5-dimethoxybenzoyl)-indoline
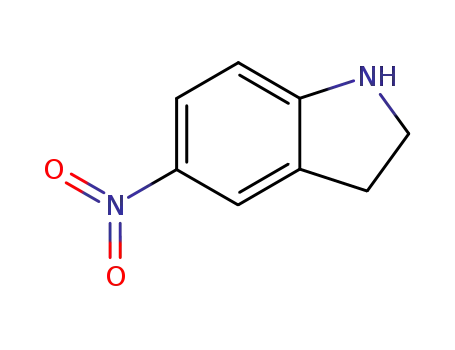
5-nitro-2,3-dihydro-1H-indole
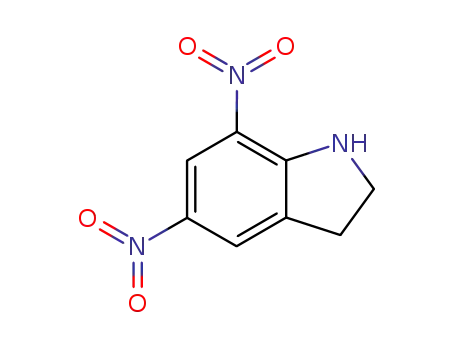
5,7-dinitroindoline
CAS:69249-61-2
CAS:2618-96-4
CAS:577-19-5