Your Location:Home >Products >Functional intermediates >2618-96-4
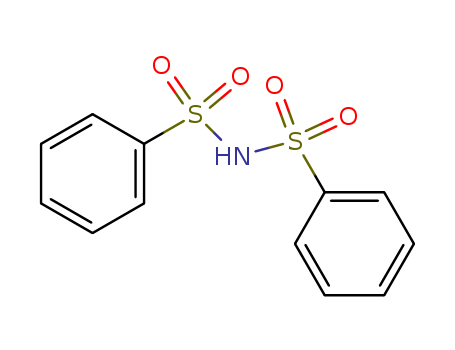

Product Details
Chemical Properties
White crystalline
Flammability and Explosibility
Notclassified
InChI:InChI=1/C12H11NO4S2/c14-18(15,11-7-3-1-4-8-11)13-19(16,17)12-9-5-2-6-10-12/h1-10,13H
A mild and efficient protocol for the copper(I)-catalyzed C4-H sulfamidation of 1-naphthylamine derivatives with diphenylsulfonimide (NHSI) was explored at room temperature, affording the desire produces in moderate to good yields. The control experiments indicated that this visible-light-promoted reaction might proceed via a single-electron-transfer process. In addition, preliminary DFT studies for the intermediates in the catalytic cycle were also explored, indicating that the C4 site in the naphthyl ring is the most likely electrophilic reactive site and providing some exact basis for the plausible mechanism.
Catalytic hydroamination of the readily available alkenes is among the most straightforward means to construct diverse alkyl amines. To this end, the facile access to both regioselectivity, i.e., Markovnikov or anti-Markovnikov hydroamination, with minimum reaction-parameter alternation, remains challenging. Herein, we report a cobalt-catalyzed highly selective and divergent Markovnikov and anti-Markovnikov hydroamination of alkenes, in which the switch of regioselectivity is achieved simply by the variation of the addition sequence of 9-BBN.
An unprecedented N-demethylation of N-methyl amides has been developed by use of N-fluorobenzenesulfonimide as an oxidant with the aid of a copper catalyst. The conversion of amides to carbinolamines involves successive single-electron transfer, hydrogen-atom transfer, and hydrolysis, and is accompanied by formation of N-(phenylsulfonyl)benzenesulfonamide. Carbinolamines spontaneously decompose to N-demethylated amides and formaldehyde, because of their inherent instability.
N -Sulfonylformamidines were synthesized from N -sulfonylsulfonamides by reacting with p -toluenesulfonyl chloride (TsCl) and N, N - disubstituted formamides. In this reaction, it was expected that mixing TsCl with the N, N -disubstituted formamide would generate an iminium salt (Vilsmeier reagent). The reaction avoids the use of metal catalysts and hazardous reagents, and the desired N -sulfonylformamidines were obtained in 60% to quantitative yields.
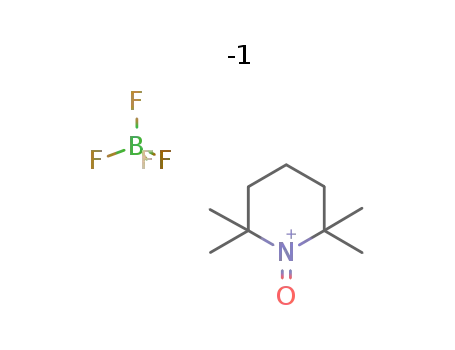
2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate

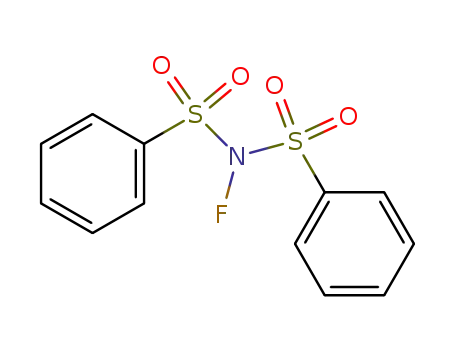
N-fluorobis(benzenesulfon)imide

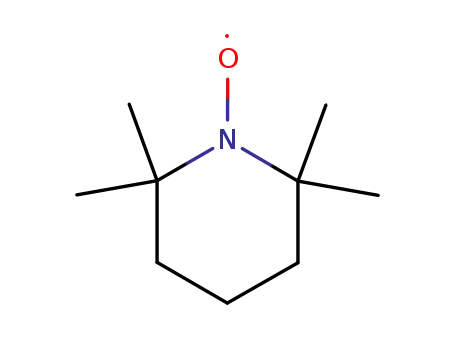
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

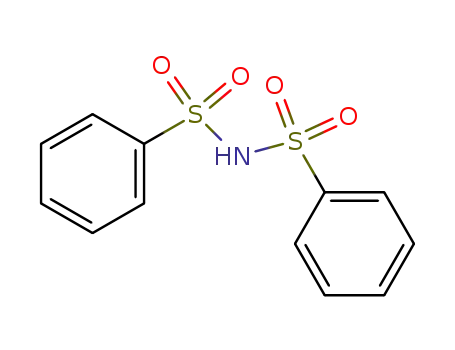
dibenzenesulfonamide
| Conditions | Yield |
|---|---|
|
With
1,1,1,2,2,2-hexamethyldisilane; copper(l) chloride;
In
acetonitrile;
at 70 ℃;
for 1h;
Schlenk technique;
Inert atmosphere;
|
100% 86% |

2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate


N-fluorobis(benzenesulfon)imide


dibenzenesulfonamide
| Conditions | Yield |
|---|---|
|
With
copper(l) chloride;
In
acetonitrile;
at 70 ℃;
for 1h;
Schlenk technique;
Inert atmosphere;
|
62% |
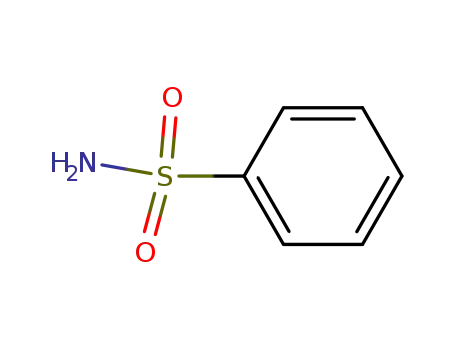
benzenesulfonamide
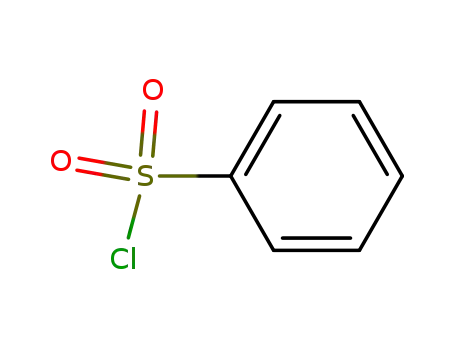
benzenesulfonyl chloride
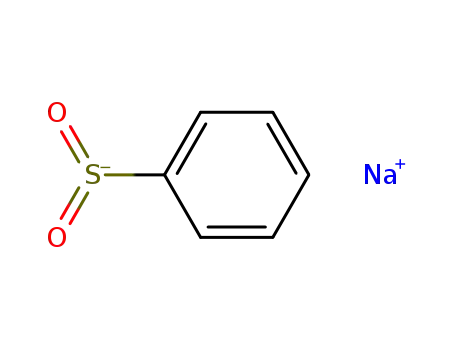
sodium benzenesulfonate
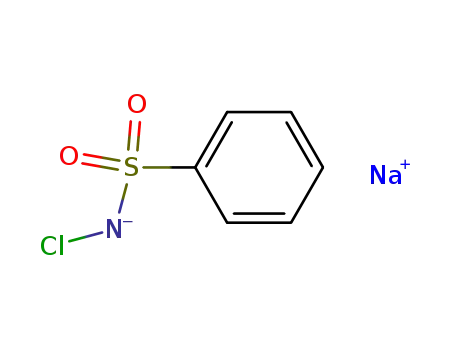
chloramine-B
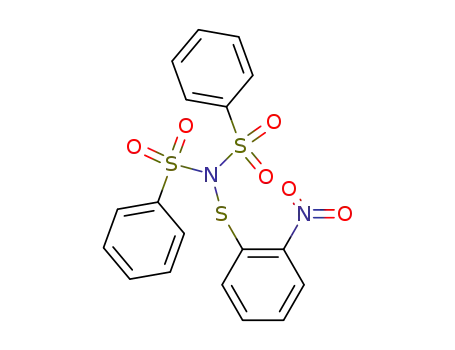
N,N-Bis-
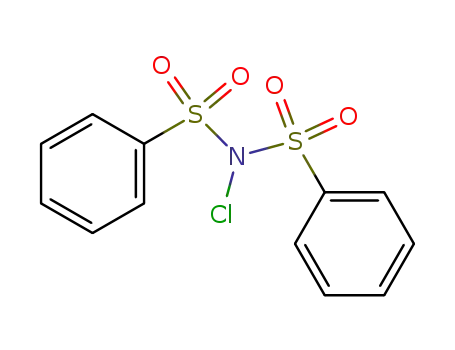
N-chloro-N-(benzenesulfonyl)benzenesulfonamide
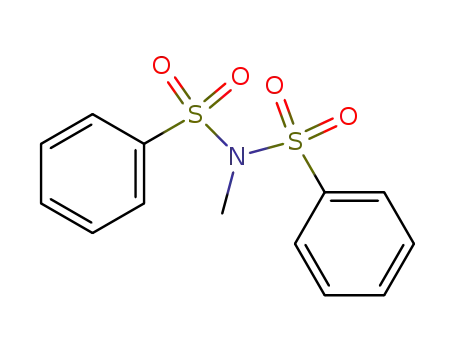
N-(benzenesulfonyl)-N-methyl-benzenesulfonamide
di-tert-butyl nitroxide
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09
CAS:65344-26-5
CAS:5315-79-7
CAS:496-15-1