Your Location:Home >Products >OLED intermediates >Thiophenes >145543-83-5


Product Details
Chemical Properties
Colorless to yellow to brown liquid
InChI:InChI=1/C12H19BrS/c1-2-3-4-5-6-7-8-11-9-10-14-12(11)13/h9-10H,2-8H2,1H3
Poly(3,3″-dioctyl-2,2′5′,2″-terthiophene), obtained from its corresponding monomer by oxidative polymerization with FeCl 3, has been fractionated into five fractions of reduced polydispersity, covering the Mn range from 1.50 kDa to 10.50 kDa (measured vs. polystyrene standards). The effect of Mn on spectroscopic, electrochemical, spectroelectrochemical and electrical transport properties has been investigated. Fractions of growing Mn show an increasing bathochromic shift of the band originating from the π-π* transition in the neutral polymer with the appearance of a clear vibrational structure for the two highest molecular fractions. The onset of oxidative doping determined from the cyclic voltammogram shifts towards lower potentials with increasing molecular weight. A similar trend is observed for doping induced near infrared bands, which shift towards lower energies (higher wavelengths) with increasing molecular weight and appear at lower potentials in spectroelectrochemical experiments. Finally, a comparison of the FET mobility in two transistors fabricated under identical conditions from polymer fractions differing in their molecular weight shows that a ca. fourfold increase of M n (from 2.40 kDa to 10.50 kDa) results in a two orders of magnitude increase in the carriers' mobility (from μsat = 4 × 10 -5 cm2 V-1 s-1 to μsat = 2 × 10-3 cm2 V-1 s-1). The obtained results underline the importance of the control of the macromolecular parameters in the preparation of electronic and electrochemical devices from poly(3,3″-dioctyl-2,2′5′,2″-terthiophene). The Royal Society of Chemistry 2006.
A series of dialkynyl-functionalized oligo(3-octylthiophene)m (m = 1-7 and 9, where m is the number of thiophene units) were polymerized together with 2,6-bis(pyrazole)pyridine (BPP) to get alternating copolymers (P1-P7 and P9) with the number-average molecular weights (Mn) in the range of 5.3-11 kDa. These copolymers were further self-assembled into solid microspheres. The optical emission range of these copolymers were fine-tuned from blue to red and white by changing the conjugation length of thiophene oligomers (band gap approximately from 2.1 to 1.67 eV) and coordination of metals like Eu3+ and Tb3+ in both the solution and solid states. A white color was obtained in solution, thin film, and microsphere states with CIE (Commission Internationale de l'Eclairage) coordinates close to the values of standard white color.
Two alternating medium band gap conjugated polymers (PBDT-TPTI and PDTBDT-TPTI) derived from 4,8-bis(4,5-dioctylthien-2-yl)benzo[1,2-b:4,5-b′]dithiophene (BDT-T) or 5,10-bis(4,5-didecylthien-2-yl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene (DTBDT-T) with pentacyclic aromatic lactam of N,N-didodecylthieno[2′,3′:5,6]pyrido[3,4-g]thieno[3,2-c]-iso-quinoline-5,11-dione (TPTI), are synthesized and characterized. The comparative investigation of the photostabilities of the copolymers revealed that the PDTBDT-TPTI film exhibited the comparable photostability in relative to P3HT. Meanwhile, the inverted photovoltaic cells (i-PVCs) from the blend films of PBDT-TPTI and/or PDTBDT-TPTI with PC71BM, in which poly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctylfluorene)] were used as cathode modifying interlayer, presented higher power conversion efficiencies (PCEs) of 5.98% and 6.05% with photocurrent response ranging from 300 nm to 650 nm in contrast with the PCEs of 4.48% for the optimal inverted PVCs from P3HT/PC71BM under AM 1.5 G 100 mW/cm2. The PCEs of the i-PVCs from PBDT-TPTI and PDTBDT-TPTI were improved to 7.58% and 6.91% in contrast to that of 0.02% for the P3HT-based i-PVCs, and the photocurrent responses of the devices were extended to 300–792 nm, when the ITIC was used as electron acceptor materials. The results indicate that the PBDT-TPTI and PDTBDT-TPTI can be used as the promising alternatives of notable P3HT in the photovoltaic application.
The 1H and 13C signals of the aromatic region of monodisperse regioregular head-to-tail (HT) oligo(octylthiophene)s ranging from the dimer to the hexamer were assigned in CDCl3 and THF-d8. The linear dependence of proton chemical shifts on the reciprocal number of thiophenes was demonstrated in both solvents. Weak signals surrounding the main peak in the spectrum of the regioregular HT poly(octylthiophene) were assigned in CDCl3 and THF-d8 in the light of results obtained for the hexamer. In particular, the end of chain protons of the polymer could be assigned. Spin-lattice relaxation times (T1) of aromatic protons of the hexamer were measured in CDCl3 and THF-d8. We observed that T1 depended on the position of the proton along the main chain. This result was interpreted in terms of molecular motions. Copyright
New star-shaped non-fullerene acceptors (5Z,5′Z,5′′Z)-5,5′,5′′-((benzo[1,2-b : 3,4-b′ : 5,6-b′′]trithiophene-2,5,8-triyltris(4-octylthiophene-5,2-diyl))tris(methaneylylidene))tris(3-octyl-2-thioxothiazolidin-4-one) (1: BTT-OT-ORD) and 2,2′,2′′-((5Z,5′Z,5′′Z)-((benzo[1,2-b : 3,4-b′ : 5,6-b′′]trithiophene-2,5,8-triyltris(4-octylthiophene-5,2-diyl))tris(methaneylylidene))tris(3-octyl-4-oxothiazolidine-5,2-diylidene))trimalononitrile (2: BTT-OT-OTZDM) with a benzotrithiophene core, alkyl-thiophen units, and acceptor units were designed and synthesized. The HOMO-LUMO levels of 1 and 2 were determined by photoemission spectroscopy and UV-Vis absorption spectroscopy. Binary blend and ternary blend bulk heterojunction (BHJ) organic solar cells with non-fullerene acceptors 1 and 2 were fabricated with the inverted device structures of glass/ITO/ZnO/active_layer/MoO3/Ag. Both binary blend BHJ solar cells with 1 and 2 show lower JSC and larger VOC values than P3HT : PCBM solar cells. On the other hand, ternary blend BHJ organic solar cells, including 10 % of 1, exhibited a larger power conversion efficiency than P3HT : PCBM solar cells because the JSC value was largely improved.
Bolaamphiphilies with D-A-D type π-conjugated rigid cores composed by thiophenes as donors (D) and benzothiadiazole (BTD) as central acceptor (A) have been synthesised. Their self-assemblies and photophysical properties were investigated by polarising optical microscope, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. Such compounds can self-assemble into honeycomb cylinder mesophases with Colhex?/p6mm and Colsqu/p4mm lattices in their pure states as well as organogels with different morphologies in organic solvents. Their absorption spectra cover nearly the entire visible light range and their band gaps are relatively low. Tetrathiophene BTD based bolaamphilphiles (BT4/n) with higher D/A ratios than the bisthiophene BTD bolaamphilphiles (BT2/n) can self-assemble into more ordered nanostructures in both bulk states and solution. Both the absorption and emission peaks of BT4/n are strongly red shifted. The influence of the molecular conformation, the conjugated core length, as well as the D/A ratio on the self-assemble and photophysical characteristics of such D-A-D bolaamphiphiles are discussed.
In this work, we present a series of newly synthesized conjugated oligothiophene derivatives, with different numbers of central thiophene units, and different donor/acceptor architectures. Electrochemical and spectroscopic data have also been reported. We used thiophene or bithiophene as central donor core units, 3-octylthiophenes as π-bridge and solubilizing sub-units, and ethyl cyanoacetate or rhodanine moieties as acceptor end groups, in order to get D-π-A and A-π-D-π-A molecular architectures. The length of the synthesized oligothiophenes ranges from three to eight thiophene units, a variety that is sufficient to put in evidence different optical and electrochemical characteristics as well as semiconducting characteristics. Oligothiophene compounds can be regarded not only as models for the study of structure-property relationships relative to polythiophenes, but also they present a large number of applications in the field of organic electronics (i.e.: as donors in bulk-heterojunction solar cells and hole-transporting layer materials in perovskite solar cells, among others).
The present invention relates to a triphenylamine compound, to a polymer thereof and to an electrochromic device comprising the same, and specifically, to a triphenylamine compound represented by the chemical formula (1), to a polymer thereof and to an electrochromic device comprising the same. In the chemical formula (1), R, R1, R2, R3, R4, and R5 are as defined in claim 1. The present invention also provides a triphenylamine-based polymer capable of forming a thin film having uniform surface and exhibiting excellent reversibility in the electrochromic characteristics.COPYRIGHT KIPO 2018

3-octylthiophene

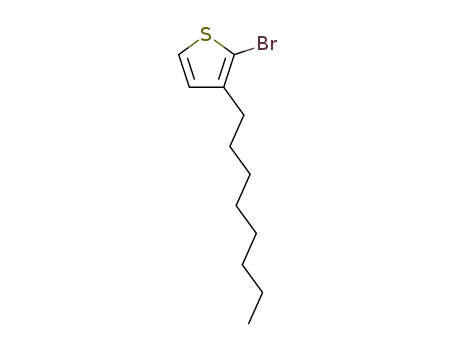
2-bromo-3-octylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
at 15 ℃;
for 2h;
|
98% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
Inert atmosphere;
|
98% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
97.9% |
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.166667h;
|
94.2% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
|
94.6% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 5 - 25 ℃;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
Ambient temperature;
|
91% |
|
3-octylthiophene;
With
N-Bromosuccinimide;
In
chloroform;
at 20 ℃;
for 4h;
With
N-Bromosuccinimide;
In
chloroform;
for 4h;
Heating;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12h;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Cooling with ice;
|
89% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Cooling with ice;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
|
85% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
82% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 3h;
|
81% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 2h;
Ambient temperature;
|
79% |
|
With
bromine;
In
tetrachloromethane;
at -20 ℃;
for 2h;
|
75% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 1.25h;
|
65.8% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
60% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
40% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
|
|
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
Inert atmosphere;
|
14.4 g |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
|
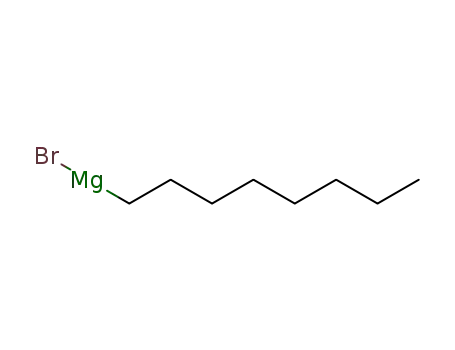
octylmagnesium bromide


2-bromo-3-octylthiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 61 percent / Ni(dppp)Cl2 / diethyl ether / 35 °C
2: 40 percent / Br2 / acetic acid / 0.5 h / 0 °C
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); bromine;
In
diethyl ether; acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 25 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 0.25 h / 0 - 5 °C / Inert atmosphere
2: N-Bromosuccinimide; acetic acid / chloroform / 0.5 h / 0 °C / Inert atmosphere
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); acetic acid;
In
diethyl ether; chloroform;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 0 - 5 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / 0 - 20 °C / Inert atmosphere
2: N-Bromosuccinimide / tetrahydrofuran / Inert atmosphere
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 20 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
|

3-octylthiophene
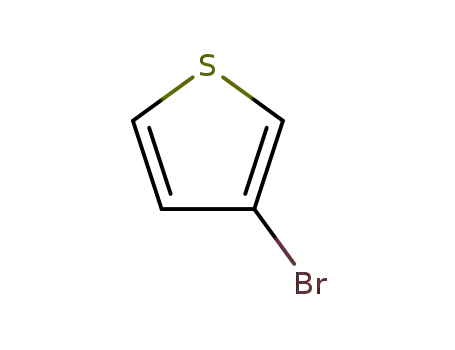
3-Bromothiophene
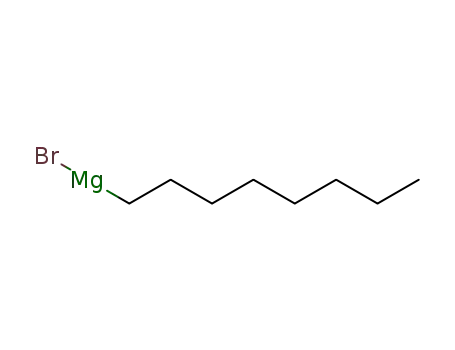
octylmagnesium bromide
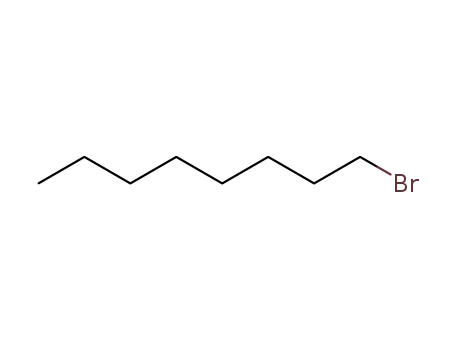
1-bromo-octane

3,3″-dioctyl-2,2′:5′,2″-trithiophene
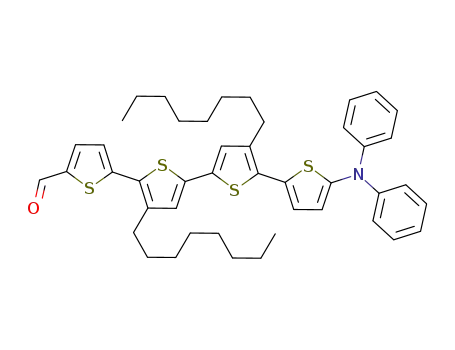
3',4''-dioctyl-5'''-(diphenylamino)-2,2':5',2'':5'',2'''-quaterthiophene-5-carboxaldehyde
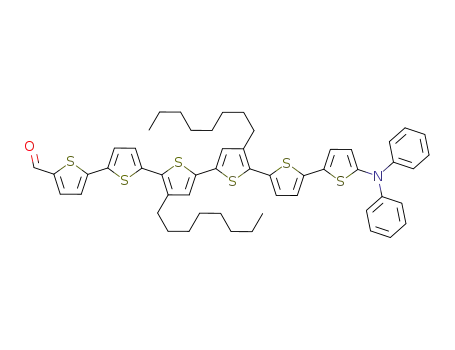
3'',4'''-dioctyl-5'''''-(diphenylamino)-2,2':5',2'':5'',2''':5''',2'''':5'''',2'''''-sexithiophene-5-carboxaldehyde
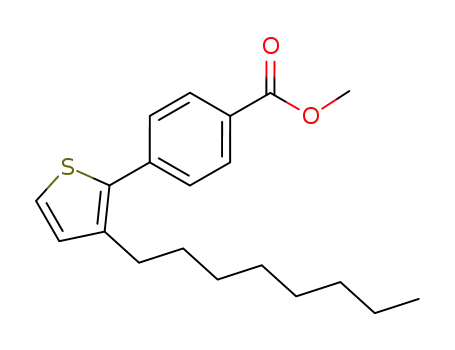
methyl 4-(3-octylthiophen-2-yl)benzoate
CAS:855738-89-5
CAS:400607-31-0
CAS:149703-84-4
CAS:65016-62-8