Your Location:Home >Products >OLED intermediates >Fluorenes >400607-31-0
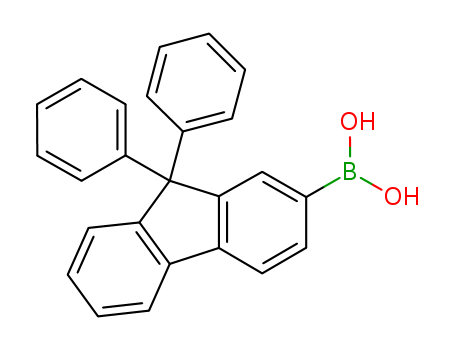

Product Details
Uses
B-(9,9-Diphenyl-9H-fluoren-2-yl)-boronic Acid can be used to synthesize pyrrole/polycyclic aromatic units. It is a potential electroluminescent material.
The invention relates to an electron donor compound, a light-emitting device, a preparation method of the light-emitting device and a display device. The electron donor compound has the following structural groups: each R1 independently selected from hydrogen, a trimethylsilyl group, a cyclohexyl group, a 3-pentyl group, a 4-(9,9'-spirobifluorene) group, a 2-(9,9'-diphenyl fluorene) group or a tetraphenyl vinyl group, wherein each R1 is not hydrogen at the same time. When the electron donor compound provided by the invention is used as an electron donor material to be applied to an interface heterojunction exciplex system, the original contact point of an electron donor and an electron acceptor is isolated by a non-hydrogen substituent, so that electrons and holes are separated in space, according to the invention, electrons of the electron acceptor layer can be prevented or hindered from easily moving into the electron donor layer, so that the electrification problem of quantum dots is avoided or relieved, and the efficiency and the service life of the light-emitting device are improved.
The invention provides an organic electroluminescence compound. The organic electroluminescence compound is shown in a chemical formula (I), in the previous formula, A and A' are respectively and independently R0 or R1, R0 is 9, 9'- spirobifluorene-2'-yl, R1 can be selected from substituted or unsubstituted aromatic base, heterocyclic aromatic base or heterocyclic base and the like; at least one of A and A' is R0. The compound in the chemical formula (I) is an electron transport material, so that the luminescence efficiency and the heat stability of an electroluminescence element can be improved. The invention also provides the organic electroluminescence element prepared by the organic electroluminescence compound.
This invention provides an organic electroluminescent compound, which is represented by the following chemical formula (I). In the above formula, A and A' are each independently R0 or R1, and R0 is 9,9'-spirobifluorene-2'-yl, R1 may be selected from substituted or unsubstituted aryl, heteroaryl or heterocyclyl; and at least one of A and A' must be R0. The compound of the formula (I) may be an electron transport material and thereby improve the luminous efficiency and thermal stability of a light emitting element. This invention further provides an organic electroluminescent element made from the organic electroluminescent compound.
The invention provides a composition comprising at least one fluorene substituted triazine derived compound of Formula 1, as described herein, and electronic devices containing the same. Such devices have improved efficiency and better driving voltage.
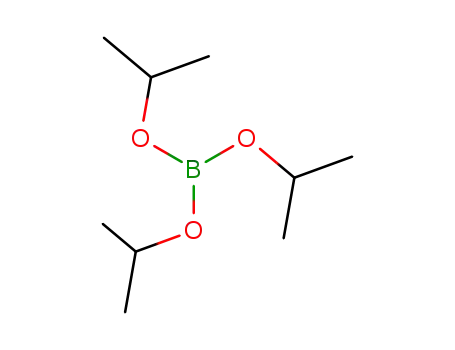
Triisopropyl borate

water


2-bromo-9,9-diphenyl-9H-fluorene

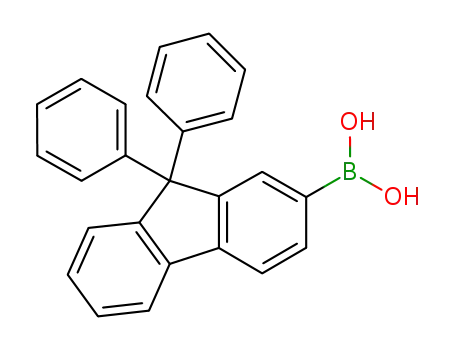
(9,9-diphenyl-9H-fluoren-2-yl)boronic acid
| Conditions | Yield |
|---|---|
|
Triisopropyl borate; 2-bromo-9,9-diphenyl-9H-fluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 15h;
Inert atmosphere;
water;
In
tetrahydrofuran;
|
78% |
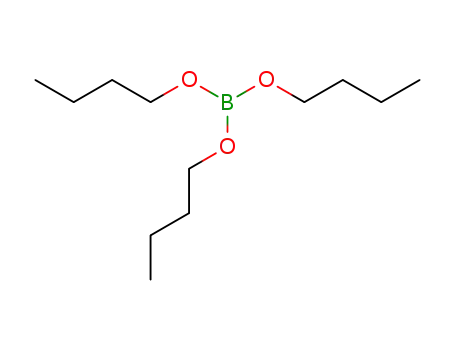
boric acid tributyl ester


2-bromo-9,9-diphenyl-9H-fluorene


(9,9-diphenyl-9H-fluoren-2-yl)boronic acid
| Conditions | Yield |
|---|---|
|
boric acid tributyl ester; 2-bromo-9,9-diphenyl-9H-fluorene;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at -10 ℃;
for 2h;
Inert atmosphere;
|

boric acid tributyl ester

2-bromo-9,9-diphenyl-9H-fluorene
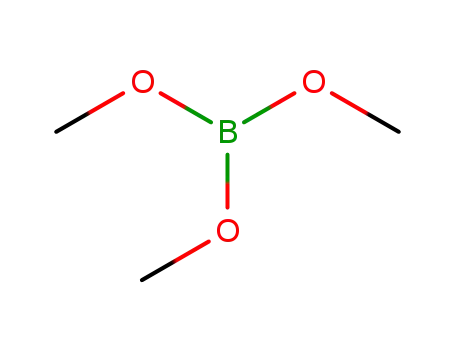
Trimethyl borate

water

1-ethyl-3,4-bis(naphthalen-2-yl)-2,5-bis(4-(9,9-diphenyl-9H-fluoren-2-yl)phenyl)-1H-pyrrole
CAS:787618-22-8
CAS:393841-81-1
Molecular Formula:C33H31Br
Molecular Weight:507.5