Your Location:Home >Products >Organic phosphines >Tert-butyl phosphines >32673-25-9
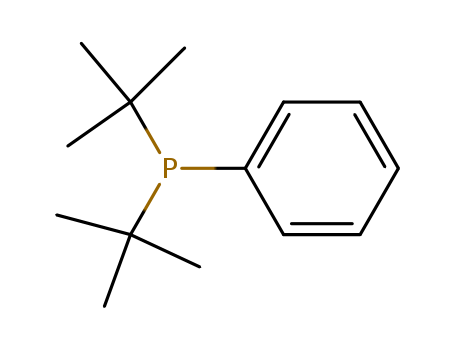

Product Details
Uses
Di-tert-butylphenylphosphine is used as a catalyst in the cross-coupling of secondary organotrifluoroborates with aryl chlorides and bromides.
InChI:InChI=1/C14H23P/c1-13(2,3)15(14(4,5)6)12-10-8-7-9-11-12/h7-11H,1-6H3
The kinetics of quinuclidine displacement of BH3 from a wide range of Lewis base borane adducts have been measured. Parameterization of these rates has enabled the development of a nucleofugality scale (NFB), shown to quantify and predict the leaving group ability of a range of other Lewis bases. Additivity observed across a number of series R′3-nRnX (X = P, N; R′ = aryl, alkyl) has allowed the formulation of related substituent parameters (nfPB, nfAB), providing a means of calculating NFB values for a range of Lewis bases that extends far beyond those experimentally derived. The utility of the nucleofugality parameter is explored by the correlation of the substituent parameter nfPB with the hydrolyses rates of a series of alkyl and aryl MIDA boronates under neutral conditions. This has allowed the identification of MIDA boronates with heteroatoms proximal to the reacting center, showing unusual kinetic lability or stability to hydrolysis.
The reaction of (tBu)2ArP (1a-h), where the para position of the Ar group contains electron-donating or electron-withdrawing groups, with sulfated zirconium oxide partially dehydroxylated at 300 °C (SZO300) forms [(tBu)2ArPH][SZO300] (2a-h). The equilibrium binding constants of 1a-h to SZO300 are related to the pKa of [(tBu)2ArPH]; R3P that form less acidic phosphoniums (high pKa values) bind stronger to SZO300 than R3P that form more acidic phosphoniums (low pKa values). These studies show that Br?nsted acid sites on the surface of SZO300 are not superacidic.
The invention relates to a method for preparing phosphine benzene apperception compound, the bromine compound is made with the magnesium reaction of Grignard reagent, then using four (triphenylphosphine) palladium-catalyzed bromophenylacetic compound phosphine chlorination reaction with the Grignard reagent. The specific method is, under the protection of inert gas, the bromo compound is made with the magnesium chips and organic solvent Grignard reagent, reflux 2 the [...] 10 hours; joined four at room temperature (triphenylphosphine) palladium, stirring 10 minutes to 3 hours, chlorinated room temperature instillment phosphine composition, reflux reaction the 1 [...] 10 hours; in the ice water bath in the fluid adds by drops full and weak acid to the reaction under weak alkali salt-water solution, liquid, organic phase exsolution, alcohol solvent crystallization, filtering to obtain compound phosphine benzene. The preparation method of this invention not only greatly improves manufacturing yield, and after treatment is simple, the process of repeated washing with ammonia water, the manufacturing process is simplified, is beneficial for large-scale industrial production. (by machine translation)
Novel catalytic reductions of tertiary and secondary phosphine oxides to phosphines have been developed. Using tetramethyldisiloxane (TMDS) as a mild reducing agent in the presence of copper complexes, PO bonds are selectively reduced in the presence of other reducible functional groups (FGs) such as ketones, esters, and olefins. Based on this transformation, an efficient one pot reduction/phosphination domino sequence allows for the synthesis of a variety of functionalized aromatic and aliphatic phosphines in good yields.
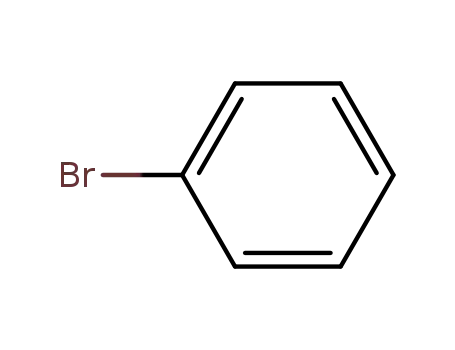
bromobenzene

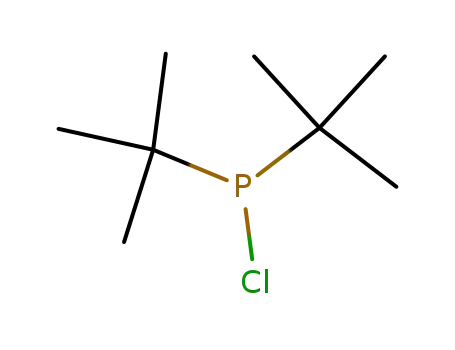
di(tert-butyl)chlorophosphine

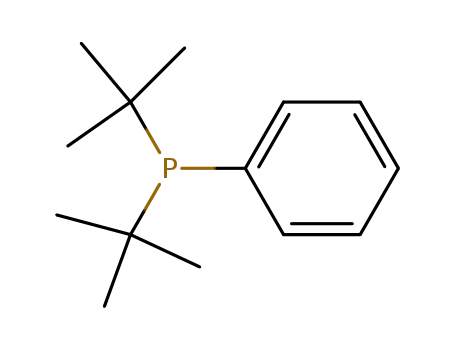
di(tertbutyl)phenylphosphine
| Conditions | Yield |
|---|---|
|
bromobenzene;
With
magnesium;
In
tetrahydrofuran;
for 2h;
Inert atmosphere;
Reflux;
di(tert-butyl)chlorophosphine;
With
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran;
at 20 ℃;
for 3h;
Reflux;
Inert atmosphere;
|
94.7% |
|
With
tert.-butyl lithium;
In
diethyl ether;
1.) -40 deg C, 2.) r.t. to reflux;
|
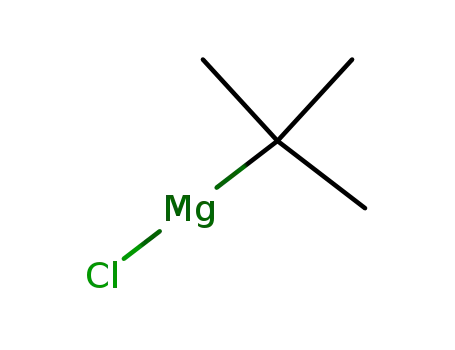
tert-butylmagnesium chloride

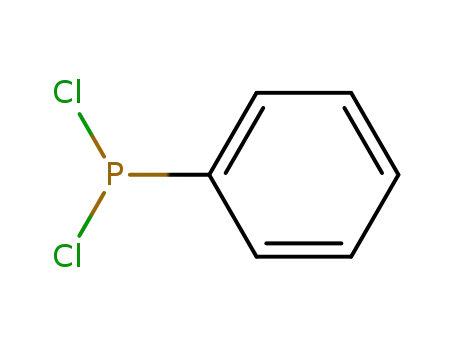
Dichlorophenylphosphine


di(tertbutyl)phenylphosphine
| Conditions | Yield |
|---|---|
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; hexane;
at -20 - 20 ℃;
for 3.5h;
|
71% |
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; toluene;
at 20 ℃;
for 0.5h;
Product distribution / selectivity;
|
93.1 %Spectr. |
|
With
lithium bromide;
copper(l) iodide;
In
1,4-dioxane; diethyl ether;
at 20 ℃;
for 0.5h;
Product distribution / selectivity;
|
91.7 %Spectr. |
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; hexane;
at 20 ℃;
for 0.5h;
Product distribution / selectivity;
|
98.7 %Spectr. |
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; hexane;
at 20 ℃;
for 0.5 - 3.5h;
Quantum yield;
|
61 - 94.6 %Spectr. |
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; hexane;
at -40 - 50 ℃;
for 1.83333h;
Kinetics;
|
6 - 95.8 %Spectr. |
|
With
lithium bromide;
copper(I) bromide dimethyl sulphide complex;
In
diethyl ether; hexane;
at -40 - 40 ℃;
for 0.5h;
Kinetics;
|
90.4 - 95 %Spectr. |
|
With
lithium bromide;
copper(I) bromide dimethyl sulphide complex;
In
diethyl ether; hexane;
at 20 ℃;
for 1 - 2h;
Quantum yield;
|
88 - 90 %Spectr. |
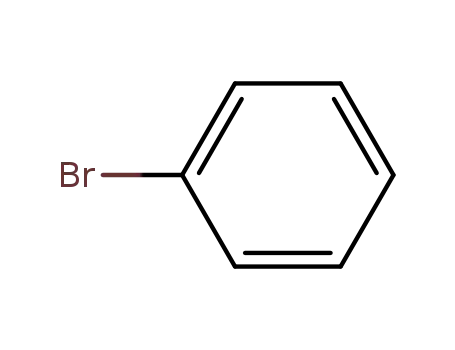
bromobenzene

di(tert-butyl)chlorophosphine
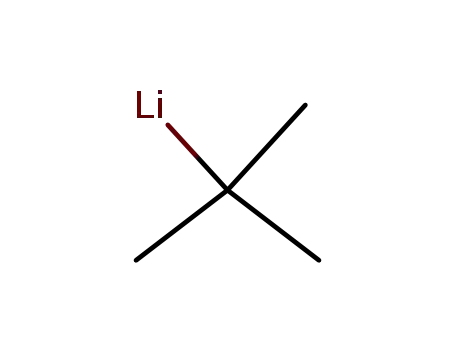
tert.-butyl lithium

Dichlorophenylphosphine
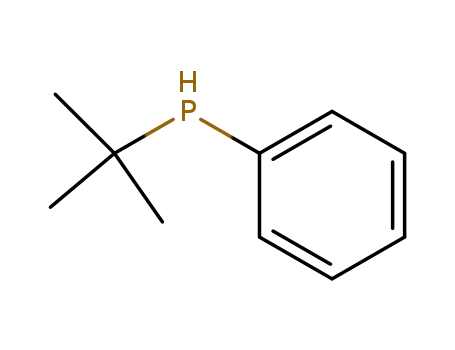
t-butylphenylphosphine
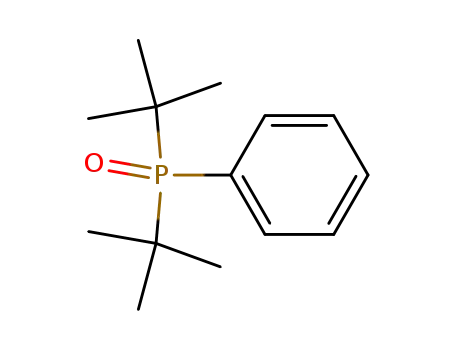
bis(tert-butyl)phenylphosphine oxide
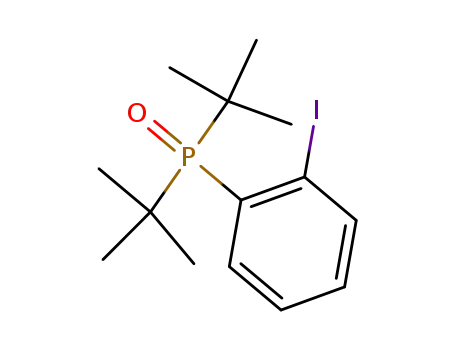
1-(Di-tert-butyl-phosphinoyl)-2-iodo-benzene
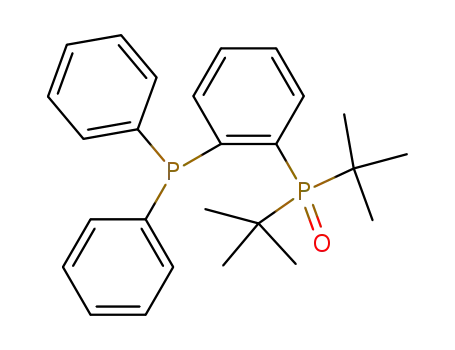
Ph2C6H4POtBu2
CAS:90-41-5
CAS:76186-72-6
CAS:131274-22-1
Molecular Formula:C12H28BF4P
Molecular Weight:290.13
CAS:6002-34-2