Your Location:Home >Products >Functional intermediates >5315-79-7
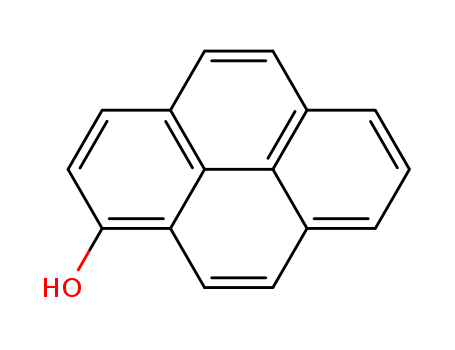

Product Details
Chemical Properties
Pale Yellow Solid
Uses
Found in humane urine after exposure to coal tar and a coal derived product.
Uses
1-Hydroxypyrene is suitable for use in following studies:To investigate the fast detection and quantification of 1-hydroxypyrene in tissue extracts from Nereis diversicolor exposed to sediment-associated pyrene by a simple fluorometric method.To investigate the effects of genetic polymorphisms of the cytochrome P450 1A1 (CYP1A1) and 2E1 (CYP2E1) and glutathione S-transferases mu (GSTM1) and theta (GSTT1) on urinary 1-hydroxypyrene and 2-naphthol levels in aircraft maintenance workers.To examine the the PAH exposure of cokery workers in an Estonian oil shale processing plant.
Uses
Found in humane urine after exposure to coal tar and a coal derived product
InChI:InChI=1/C16H10O/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,17H
Photo-Fries rearrangement reactions of 1-pyrenyl esters were investigated. Photoreaction of 1-pyrenyl benzoate in benzene generates 1-hydroxy-2-pyrenyl phenyl ketone along with 1-pyrenol. The exceptionally down field 1H NMR chemical shift of OH proton in the photoproduct indicates the existence of intramolecular hydrogen bonding. Photorearrangements of analogs that have electron-withdrawing or electron-releasing group on the phenyl ring, and related heteroaromatic carboxylates also take place to form the corresponding ketones. However, photoreactions of 1-pyrenyl aliphatic carboxylate esters do not occur. The results of spectroscopic and theoretical studies suggest the mechanistic pathway for this process is initiated by homolytic C–O bond cleavage in an aroyl group localized 1(π → π?) excited state of the 1-pyrenyl esters. The radical pair generated in this fashion then undergoes in-solvent-cage coupling to yield the 1-hydroxy-2-pyrenyl aryl ketone selectively.
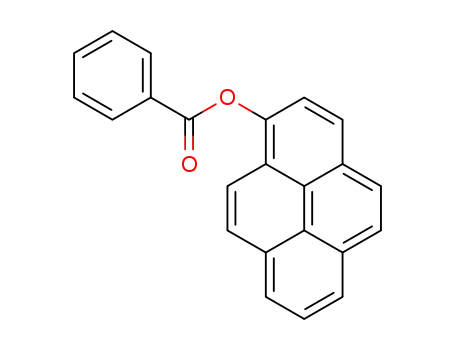
pyren-1-yl benzoate

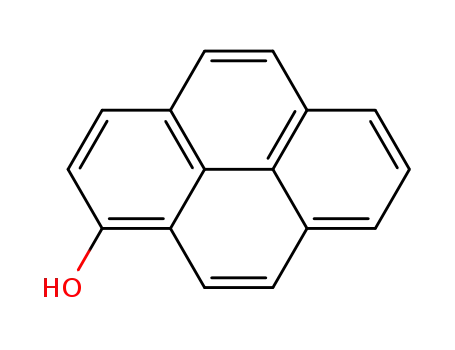
1-hydroxypyrene

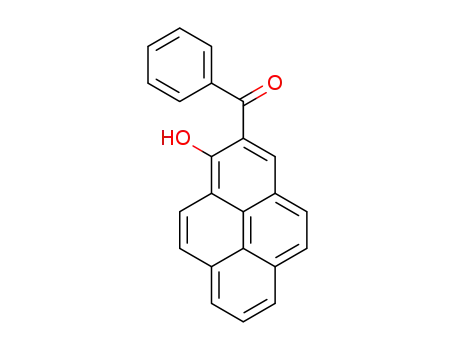
1-hydroxy-2-pyrenyl phenyl ketone

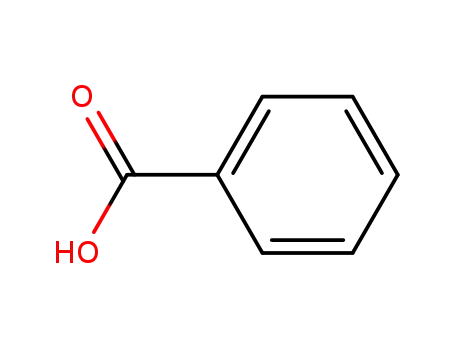
benzoic acid
| Conditions | Yield |
|---|---|
|
In
benzene;
at 20 ℃;
for 2h;
regioselective reaction;
Inert atmosphere;
Sealed tube;
Irradiation;
|
46% 14% 15% |

pyren-1-yl benzoate


1-hydroxypyrene


1-hydroxy-2-pyrenyl phenyl ketone
| Conditions | Yield |
|---|---|
|
In
acetone;
at 20 ℃;
for 2h;
regioselective reaction;
Inert atmosphere;
Sealed tube;
Irradiation;
|
61% 12% |
|
In
benzene;
at 20 ℃;
for 4h;
Solvent;
Time;
regioselective reaction;
Inert atmosphere;
Sealed tube;
Irradiation;
|
42% 18% |
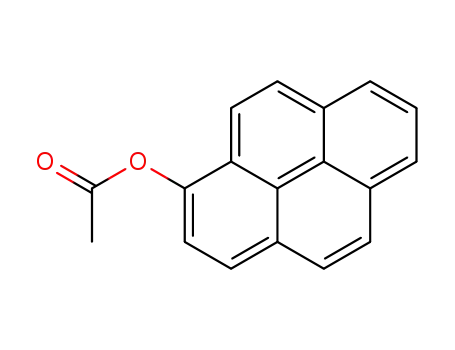
pyren-1-yl acetate
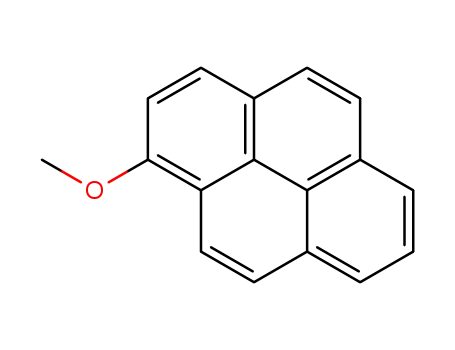
1-methoxypyrene
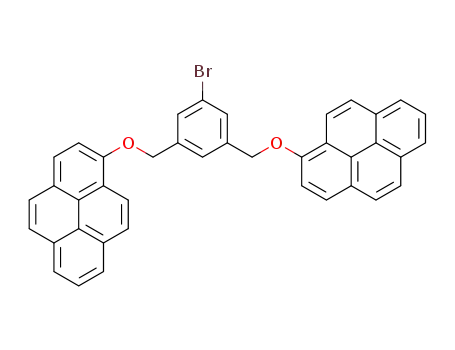
C40H25BrO2
pyrene-1,6-dione
CAS:76189-56-5
CAS:125143-53-5
CAS:2618-96-4