Your Location:Home >Products >OLED intermediates >Thiophenes >125143-53-5
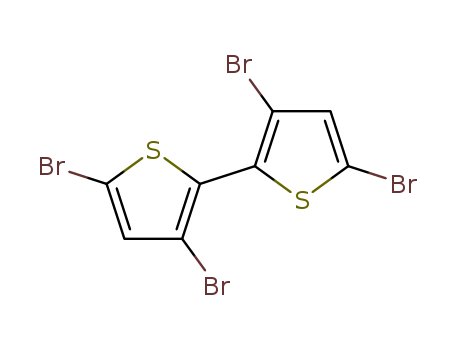

Product Details
Uses
suzuki reaction
Realizing p-channel semiconducting polymers with good hole mobility, solution processibility, and air stability is an important step forward in the chemical manipulation of charge transport in polymeric solids and in the development of low-cost printed electronics. We report here the synthesis and full characterization of the dithienosilole- and dibenzosilole-based homopolymers, poly(4,4-di-n-hexyldithienosilole) (TS6) and poly(9,9-di-n- octyldibenzosilole) (BS8), and their mono- and bithiophene copolymers, poly(4,4-di-n-hexyldithienosilole-alt-(bi)thiophene) (TS6T1, TS6T2) and poly(9,9-di-n-octyldibenzosilole-alt-(bi)thiophene) (BS8T1,BS8T2), and examine in detail the consequences of introducing dithienosilole and dibenzosilole cores into a thiophene polymer backbone. We demonstrate air-stable thin-film transistors (TFTs) fabricated under ambient conditions having hole mobilities as large as 0.08 cm2/V·s, low turn-on voltages, and current on/off ratios > 106. Additionally, unencapsulated TFTs fabricated under ambient conditions are air-stable, an important advance over regioregular poly(3-hexylthiophene) (P3HT)-based devices. Density functional theory calculations provide detailed insight into the polymer physicochemical and charge transport characteristics. A direct correlation between the hole injection barrier and both TFT turn-on voltage and TFT polymer hole mobility is identified and discussed, in combination with thin-film morphological characteristics, to explain the observed OTFT performance trends.
Novel blue-light-emitting materials based on ZnO and 2,2′-bithiophene-5,5′-dicarboxylic acid (DTDA), 4′,3″-dipentyl-5,2′: 5′,2″: 5″,2?-quaterthiophene-2,5?-dicarboxylic acid (QTDA) have been prepared. The hybrid materials show that the PL λmax are at 450 and 425 nm for DTDA-ZnO and QTDA-ZnO, respectively.
Understanding the interrelationships between molecular structure and organic thin film transistor performance is key to the realization of novel organic semiconductors achieving superior device characteristics. Herein we report the synthesis, characterization, and charge-transporting properties in organic field-effect transistors (OFETs) of dithieno silole-based oligomers and copolymers having silacycloalkyl substituents. Silacyclization of the alkyl substituents on the silole silicon atom reduces steric encumbrance, contracts solid state intermolecular π-π contacts, and enhances the charge-transport capacity of the oligomers. Oligomer 3,3′-dihexylsilylene-2,2′:5, 2′′:5′,2′′′:5′′, 2′′′′:5′′′,2′′′ ′′-sexithiophene (SM5) with two Si-n-hexyl substituents is not FET-active, while the mobilities of 3,3′-cyclopentanylsilylene-2,2′: 5,2′′:5′,2′′′:5′′, 2′′′′:5′′′,2′′′ ′′′-sexithiophene (SM4) and 3,3′-cyclobutysilylene-2, 2′:5,2′′:5′,2′′′:5′′, 2′′′′:5′′′,2′′′ ′′-sexithiophene (SM3) FETs are 2.6 × 10-4 and 3.4 × 10-4 cm2/(V s), respectively. Single crystal structural data and melting point derived intermolecular packing trends parallel these FET results. Copolymers P1-P4 based on the same dithienosilole cycloalkyl cores exhibit optimized hole mobilities of 2 × 10-5, 6 × 10-4, 3 × 10-4, and 2 × 10-3 cm2/V?s, respectively, lower than that of analogous silole-containing polymers with long Si-alkyl substituents, implying that the solubilizing and self-assembly functions of Si-alkyl substituents are important for optimizing the mobility. Interestingly, copolymer [poly{[N,N′-bis(2- octyl-dodecyl)-1,4,5,8-naphthalenedicarboximide-2,6-diyl]-alt-5,5′-(3, 3′-cyclopentanylsilylene-2,2′-bithiophene (P5) films are the most ordered and exhibit a good electron mobility of 4 × 10-3 cm2/V?s after thermal annealing. All of these OFETs exhibit good ambient-stability, which is attributed to their low-lying HOMOs (>0.2 eV lower than that of P3HT), a consequence of introducing silole cores into polythiophene backbones.
Three new building blocks containing the electron-donor fused-ring motifs carbazole, dithienosilole (DTS) and dithienopyrrole (DTP) and the 2,2′:6′,2″-terpyridine electron-acceptor motif were designed and synthesized. Directed by transition metal ions, the self-assembly of the building blocks triggered polymerization to form the corresponding metallo-supramolecular polymers PCzTPY, PSiTPY and PNTPY, respectively. The UV-vis absorption maxima of the building blocks occur at long wavelengths (351, 368 and 430 nm for CzTPY, SiTPY and NTPY, respectively), which arises from intramolecular charge transfer (ICT) transitions. However, the absorption maxima of their corresponding metallo-supramolecular polymers are clearly red-shifted (to 394, 431 and 509 nm for PCzTPY, PSiTPY and PNTPY, respectively), which is caused by the incorporation of the transition metal ion into the backbones of the target polymers. Based on the above strategies, the resulting metallo-polymers exhibit reduced energy gaps, which are 2.07, 1.97 and 1.56 eV for the PCzTPY, PSiTPY and PNTPY metallo-supramolecular polymers, respectively.
A series of photoactive conjugated low band-gap copolymer (CPSB) and terpolyemrs (TPSBCz-n, n = 1 to 4) based on N-alkyl carbazole, 4,4'-dialkyl dithienosilole, and bezothiadiazole were synthesized. The copolymer and terpolymers were built with the fraction of the carbazole unit varied for 0, 2.5, 5, 10 and 25 mol%. Among the mixtures, the composition of 25 wt% of terpolymer bearing 10 mol.% of the carbazole unit, TPSBCz-3, and 75 wt% of C71-PCBM found a power conversion efficiency of 0.86% with a open-circuit voltage of 0.59 V, the short-circuit current of 4.85 mA and fill factor of 0.30 under AM 1.5 spectral illumination. Our findings suggest that terpolymer bearing low concentration of carbazole lead to a high power conversion efficiency with improved the short-circuit current due to hole mobility enhancement effect of carbazole unit. Copyright
The reactions of thiophene derivatives with benzyltrimethylammonium tetrachloroiodate, benzyltrimethylammonium tribromide, and benzyltrimethylammonium dichloroiodate in acetic acid or in acetic acid-zinc chloride under mild conditions gave chloro-, bromo-, and iodo-substituted thiophene derivatives, respectively, in satifactory yields.
-
Single-molecule junctions that are sensitive to compression or elongation are an emerging class of nanoelectromechanical systems (NEMS). Although the molecule–electrode interface can be engineered to impart such functionality, most studies to date rely on poorly defined interactions. We focused on this issue by synthesizing molecular wires designed to have chemically defined hemilabile contacts based on (methylthio)thiophene moieties. We measured their conductance as a function of junction size and observed conductance changes of up to two orders of magnitude as junctions were compressed and stretched. Localised interactions between weakly coordinating thienyl sulfurs and the electrodes are responsible for the observed effect and allow reversible monodentate?bidentate contact transitions as the junction is modulated in size. We observed an up to ≈100-fold sensitivity boost of the (methylthio)thiophene-terminated molecular wire compared with its non-hemilabile (methylthio)benzene counterpart and demonstrate a previously unexplored application of hemilabile ligands to molecular electronics.
The synthesis and characterization of two new 2,2'-bipyridine ligands containing 3-ethynylthiophene and 3,3'-diethynyl-2,2'-bithiophene substituents is presented, along with the preparation, electronic, and magnetic properties of monoand bimetallic cobalt-semiquinone valence tautomers containing these ligands.
Substituted bithiophenes are prominent fragments in functional organic materials, and they are ideally prepared via direct oxidative C-H/C-H coupling. Here, we report a novel PdII catalyst system, employing 1,10-phenanthroline-5,6-dione (phd) as the ancillary ligand, that enables aerobic oxidative homocoupling of 2-bromothiophenes and other related heterocycles. These observations represent the first use of phd to support Pd-catalyzed aerobic oxidation. The reaction also benefits from a Cu(OAc)2 cocatalyst, and mechanistic studies show that Cu promotes C-C coupling, implicating a role for CuII different from its conventional contribution to reoxidation of the Pd catalyst.
2,2′-Bithiophene and halogenated-2,2′-bithiophenes were halogenated with 2-halo-4,5-dichloropyridazin-3(2H)-one in the presence of zinc halide to give selectively the corresponding dihalo-, trihalo-, and tetrahalo-2,2′-bithiophenes involving the same or different halogens in excellent yields, respectively. Georg Thieme Verlag Stuttgart.
The selective functionalization of the 2,2'-bithiophene molecule is described.Selective alkyl substitution at the 3,3'-positions was achieved by sequential bromination of the 3,3' and 5,5' positions followed by debromination at the 5,5'-positions.The resultant 3,3'-dibromo-2,2'-bithiophene was transformed via a Grignard reaction to give a series of 3,3'-dialkyl-2,2'-bithiophenes.Finally, nitration of the active 5,5'positions gave the corresponding 3,3'-dialkyl-5,5'-dinitro-2,2'-bithiophenes.
The synthesis and physicochemical properties of a new class of thiophene/arenesilole-containing π-conjugated polymers are reported. Examples of this new polymer class include the following: poly(2,5-bis(3′,3′′-dihexylsilylene-2′,2′′-bithieno)thiophene) (TS6T1), poly(2,5′-bis(3′′,3′″-dihexylsilylene-2′′,2′″-bithieno)bithiophene) (TS6T2), poly(2,5′-bis(2′′,2′″-dioctylsilylene-1′′,1′″-biphenyl)thiophene) (BS8T1), and poly(2,5′-bis(2′′,2′″-dioctylsilylene-1′′,1′″-biphenyl)bithiophene) (BS8T2). Organic field-effect transistors (OFETs) with hole carrier mobilities as high as 0.02-0.06 cm2/V s in air, low turn-on voltages, and current on/off ratios >105-106 are fabricated using solution processing techniques with the above polymers as the active channel layer. OFETs based on this polymer class exhibit excellent ambient operational stability. Copyright
A new 2-phenylpyridine-type (ppy-type) ligand with the dithieno[3,2-b:2′,3′-d]phosphole oxide (DTPO) group has been successfully synthesized. Based on this novel ligand, three cyclometalated iridium(iii) complexes (P-Ir-P, P-Ir-T and P-Ir-C) are synthesized with symmetrical and unsymmetrical structures. Photophysical results reveal that these cyclometalated iridium(iii) complexes can show weak near-infrared (NIR) phosphorescence emission with wavelengths of 739 nm for P-Ir-P, 750 nm for P-Ir-T and 746 nm for P-Ir-C. Importantly, transient absorption characterization shows that these cyclometalated iridium(iii) complexes can exhibit strong excited state absorption in the range of ca. 520 to 700 nm, indicating their optical power limiting (OPL) potential in this wavelength range. Open-aperture Z-scan against a 532 nm laser shows their OPL ability in the order of P-Ir-P > P-Ir-C > P-Ir-T. Complex P-Ir-P shows an even better OPL ability than the state-of-the-art OPL material C60, indicating the important potential application of these cyclometalated iridium(iii) complexes as new OPL materials.
We synthesized and characterized a set of D-π-A conjugated copolymers containing thiophene π-bridge. While benzothiadiazole serves as an acceptor (A) unit, the 4,4-dialkyldithieno[3,2-b:2′,3′-d]silole (DTSi) or N-alkyldithieno[3,2-b:2′,3′-d]pyrrole (DTP) act as a donor (D) unit. The copolymers were synthesized via the commonly Stille cross-coupling reaction and exhibited molecular weights of 18.6 to 31.3 kg/mol. The main structural differences among the copolymers are the type of donor moiety (DTSi or DTP) and the position of hexyl side chains on the thiophene π-bridge units between the D and A moieties. The ultimate goal of this work is to explore the effect of three structural factors that could control the photophysical properties of polymers in order to help in the rational design of polymers having specific properties used in optoelectronic devices. The physical properties include thermal stability, photophysical, and electrochemical properties. The structural factors are (a) the power of donor moiety, (b) the position of alkyl side chain on the thiophene π-bridge, and (c) the nature of the alkyl side chain. Also, we utilized the density functional theory calculations to calculate the geometric and electronic structures. A good agreement was remarked between the experimental and theoretical findings.
The invention relates to a preparation method of a thiophene organic semiconductor material intermediate, which comprises the following steps: (1) reacting 2,2'-bithiophene with a halogenating agent to obtain tetrahalogen-substituted bithiophene; (2) selectively removing halogen at the ortho-position of tetrahalogen-substituted bithiophene to obtain meta-dihalogen-substituted bithiophene; and (3)under the protection of an inert atmosphere and in the presence of a catalyst, reacting the meta-dihalogen-substituted bithiophene with dichloro(2-ethylhexyl)octylsilane to synthesize the target product 4-(2-ethylhexyl)-4-octyl-4-silyl[3,2-b:4,5-b']dithiophene. The preparation method of the thiophene organic semiconductor material intermediate is simple in process, substances with severe corrosivity or toxicity are not used, the intermediate 3,3'-dibromo-2,2'-bithiophene does not need further reaction treatment, and the intermediate 3,3'-dibromo-2,2'-bithiophene can react with the dichloro(2-ethylhexyl)octylsilane to synthesize the target product.
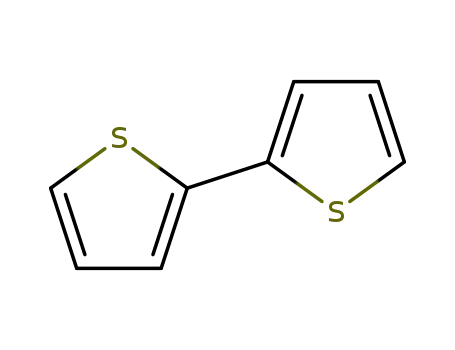
2,2'-Bithiophene

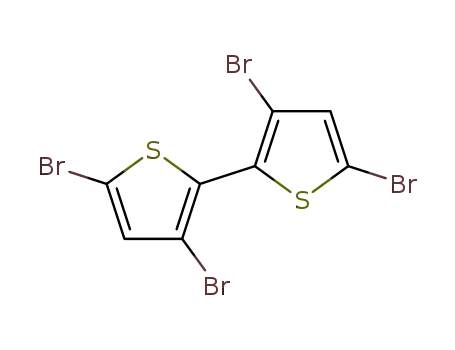
3,3',5,5'-tetrabromo-2,2'-bithiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
|
100% |
|
With
bromine;
In
chloroform; acetic acid;
at 0 - 20 ℃;
Cooling with ice;
|
100% |
|
With
bromine; acetic acid;
In
chloroform;
at 5 - 60 ℃;
Inert atmosphere;
|
98% |
|
With
bromine; acetic acid;
In
chloroform;
1.) RT, overnight, 2.) reflux, 24 h;
|
97% |
|
With
mono(N,N,N-trimethylbenzenaminium) tribromide;
zinc(II) chloride;
In
acetic acid;
at 70 ℃;
for 26h;
|
95% |
|
With
bromine; acetic acid;
In
chloroform;
1.) RT 3 h, 2.) reflux, 24 h;
|
95% |
|
With
2-bromo-3,4-dichloropyridazin-3(2H)-one; zinc dibromide;
In
dichloromethane;
at 20 ℃;
for 0.0833333h;
regioselective reaction;
|
95% |
|
With
bromine; acetic acid;
In
chloroform;
|
93% |
|
With
bromine; acetic acid;
In
chloroform;
at 0 ℃;
Reflux;
|
93% |
|
With
bromine; acetic acid;
In
chloroform;
at 0 - 20 ℃;
Heating / reflux;
|
92% |
|
With
bromine; acetic acid;
In
chloroform;
at 0 - 60 ℃;
for 2.5h;
Inert atmosphere;
|
92% |
|
With
bromine; acetic acid;
In
chloroform;
for 12h;
Reflux;
|
90% |
|
With
bromine; acetic acid;
In
chloroform;
for 13h;
Reflux;
|
90% |
|
With
bromine; acetic acid;
In
chloroform;
at 80 ℃;
for 24h;
|
90% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; acetic acid;
In
chloroform;
at 40 - 50 ℃;
for 19.5h;
|
90.6% |
|
With
bromine;
In
chloroform; acetic acid;
|
89% |
|
With
bromine; acetic acid;
In
chloroform;
for 19h;
|
87% |
|
With
bromine; acetic acid;
In
chloroform;
at 5 - 20 ℃;
for 30.5h;
Reflux;
|
78% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
|
78% |
|
With
bromine;
In
chloroform; acetic acid;
for 30.5h;
|
77% |
|
With
bromine;
In
chloroform; acetic acid;
for 24h;
Heating;
|
77% |
|
With
bromine; acetic acid;
In
dichloromethane;
Inert atmosphere;
|
77% |
|
With
bromine; acetic acid;
In
chloroform;
at 5 - 60 ℃;
for 4h;
|
70% |
|
With
bromine; acetic acid;
In
chloroform;
at 0 - 60 ℃;
for 5h;
Inert atmosphere;
|
49% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
|
|
|
With
bromine;
|
|
|
Multi-step reaction with 2 steps
1: 95.1 percent / N-bromosuccinimide / CHCl3; acetic acid / 4 h / 70 °C
2: 90 percent / N-bromosuccinimide / CHCl3; acetic acid / 4 h / 70 °C
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 50h;
Inert atmosphere;
Reflux;
|
|
|
With
hydrogen bromide; bromine;
In
water;
|
|
|
With
bromine;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 60 ℃;
for 12h;
|
|
|
With
bromine; acetic acid;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
at -20 - 0 ℃;
|
44.38 g |
|
With
bromine; acetic acid;
In
chloroform;
Reflux;
|
![3-bromo-[2,2’]bithiophenyl](/upload/2023/2/ebb58cfa-eb44-4996-a3df-bdb538dca711.png)
3-bromo-[2,2’]bithiophenyl


3,3',5,5'-tetrabromo-2,2'-bithiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 - 50 ℃;
for 20h;
|
95% |
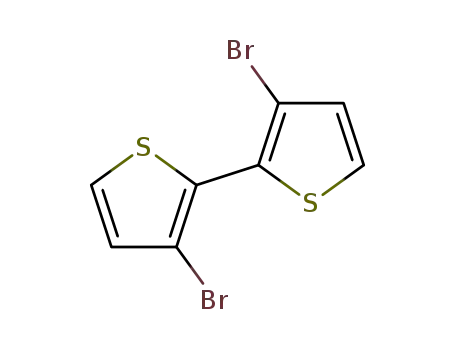
3,3'dibromo-2,2'-bithiophene

2,2'-Bithiophene
5,5'-dibromo-2,2'-bisthiophene
3,5-dibromo-[2]thienyl lithium

3,3'dibromo-2,2'-bithiophene
5,5'-dibenzoyl-3,3'-dibromo-2,2'-bithiophene
C26H10Br2N2S2
2,6-bis-(tert-butyl-dimethyl-silanyl)-4-phenyl-4H-phospholo[3,2-b;4,5-b']dithiophene
CAS:1184301-61-8
CAS:116971-10-9
CAS:119269-24-8
CAS:13029-09-9