Your Location:Home >Products >Functional intermediates >3460-18-2
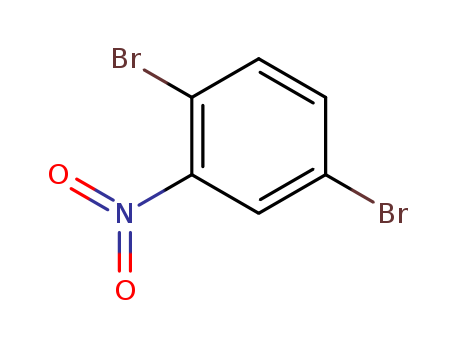

Product Details
Chemical Properties
white to light yellow crystal powder
Uses
2,5-Dibromonitrobenzene is a halogen-substituted nitrobenzenes used against Tetrahymena pyriformis due to its inhibitory and toxicity activities.
Purification Methods
It crystallises from Me2CO or EtOH. [Beilstein 5 H 250, 5 II 190, 5 III 621, 5 IV 732.]
InChI:InChI=1/C6H3Br2NO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
A series of novel naphthalimide-benzimidazoles was designed and synthesized for the first time and studied for their effect on antiproliferative activity. Some of these compounds possessed good antitumor activity towards the tested cancer cell lines. Noticeably, (diethylamino)ethyl 15 and (dimethylamino)ethyl 23 derivatives displayed superior antiproliferative activity towards human cancer cell lines with MG-MID GI50 values of 1.43 and 1.83 μM, respectively. Preliminary investigation revealed that compounds 15 and 23 might bind with ct-DNA through the intercalation mode which is responsible for potent bioactivity. Moreover, transportation behaviour indicated that these molecules could efficiently bind to and be carried by bovine albumin, and the hydrogen bonding and hydrophobic interactions played important roles in interaction with serum albumin.
The invention relates to an aromatic ring liquid crystal compound, a liquid crystal composition and an assembly suitable for a microwave region and a millimeter wave region of a high-frequency technology or an electromagnetic spectrum, and particularly a phase shifter and a microwave array antenna. The liquid crystal composition provided by the invention at least comprises a compound represented by a general formula I. The liquid crystal composition provided by the invention is good in stability, high in response speed and wide in liquid crystal phase temperature range.
A small series of amino oligo-phenylenevinylenes (OPVs) were successfully synthesized from their nitro-analogs in a rapid, simple, and highly efficient fashion employing a sodium sulfide/pyridine system as a reducing agent. In this research, classic and sustainable reduction methodologies including NH4HCO2/Zn and a choline chloride/tin (II) chloride deep eutectic solvent (DES) were also evaluated, showing degradation products, incomplete reactivity, and product isolation difficulties in all cases. The straightforward Na2S/pyridine synthetic protocol proved to maintain the E-E stereochemistry of the OPV backbone that has been previously assembled by the Mizoroki–Heck cross-coupling reaction. Also, the optoelectronic properties were determined and discussed, considering the amino group insertion in these conjugated systems as a contribution for future construction of novel materials with applications in supramolecular electronics, light harvesting, and photocatalysis.
The invention discloses a preparation method of high-purity 3-bromine-9-phenylcarbazole, and belongs to the field of synthesis of organic chemicals. The method comprises the following steps: (1) conducting a nitratlon reaction on benzene dibromide to prepare an intermediate, that is, 1,4-dibromo-2-nitrobenzene; (2) enabling 1,4-dibromo-2-nitrobenzene and diphenylamine to have an Ullmann coupling reaction to prepare an intermediate, that is, (4-bromine-2-nitrobenzophenone) diphenylamine; (3) enabling the intermediate, that is, (4-bromine-2-nitrobenzophenone) diphenylamine to have a reduction reaction to prepare an intermediate, that is, (4-bromine-2-aminophenyl) diphenylamine; and (4) enabling the intermediate, that is, (4-bromine-2-aminophenyl) diphenylamine, to have a diazotization ring closing reaction to prepare 3-bromine-9-phenylcarbazole. 3-bromine-9-phenylcarbazole prepared through the method is used in the fields of OLED photoelectric materials, medicines, dyes, pesticides and the like, and is an important carbazole intermediate; the reaction operation is simple, the cost of the raw materials is low, the yield is relatively high, and the quality is high; and moreover, the process of removing dibromo in the traditional preparation method is avoided, and the production convenience is greatly improved.
The synthetic method disclosed by the invention comprises 1,4 - the following steps: the, synthesis method disclosed by. the, invention has a good industrial production prospect, 1,4 - in the prior, art that, the raw materials are firstly, 2,5 - subjected to a nitrification step and then subjected to 2,5 - an iodine, generation step to generate 2,5 - the second bromo,4-diiodo benzene 1,4 . (by machine translation)
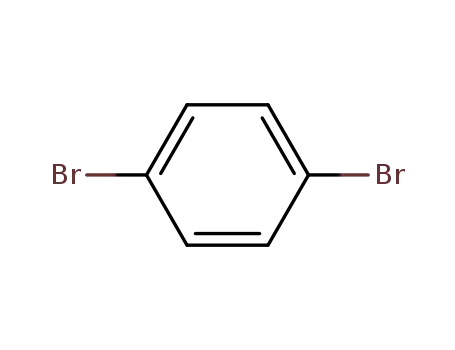
1.4-dibromobenzene

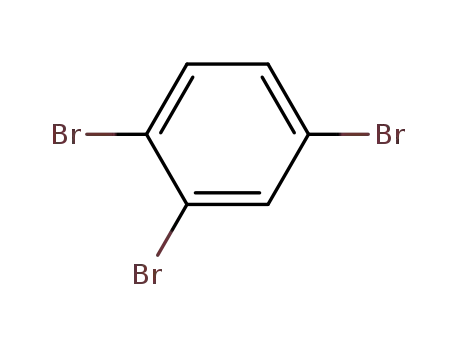
1,2,4-tribromobenzene

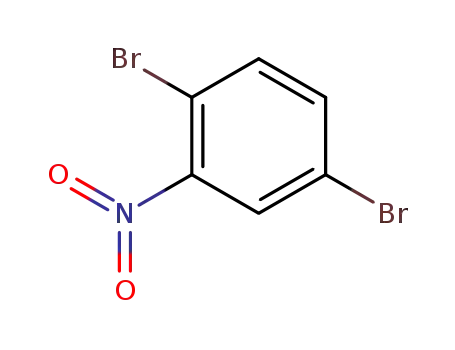
1,4-dibromo-2-nitrobenzene

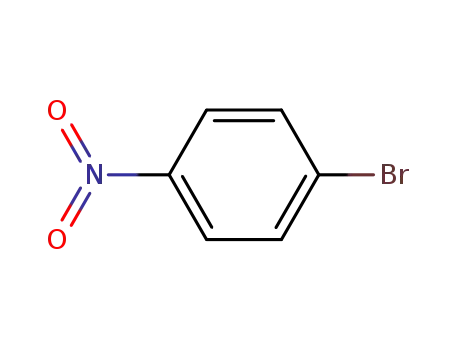
para-nitrophenyl bromide
| Conditions | Yield |
|---|---|
|
With
Nitrogen dioxide; ozone;
at 0 ℃;
for 2h;
Yield given. Yields of byproduct given;
|
|
|
With
Nitrogen dioxide; ozone;
at 0 ℃;
for 0.5h;
Yield given. Yields of byproduct given;
|
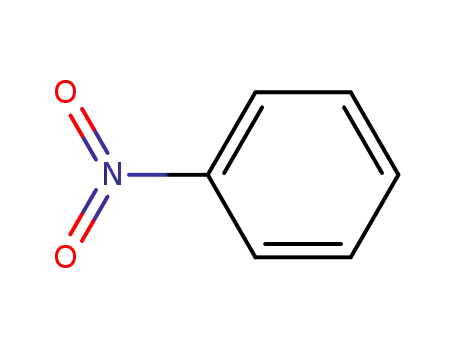
nitrobenzene


1,4-dibromo-2-nitrobenzene
| Conditions | Yield |
|---|---|
|
With
bromine fluoride;
In
ethanol; chloroform;
at -40 ℃;
for 0.75h;
|
70% |
|
With
bromine; iron(III) chloride;
at 60 ℃;
Erhitzen dann auf 80grad;
|
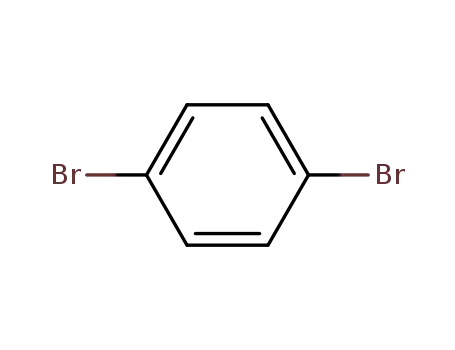
1.4-dibromobenzene
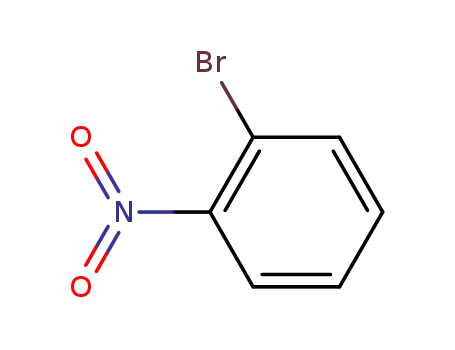
2-nitrophenyl bromide

nitrobenzene
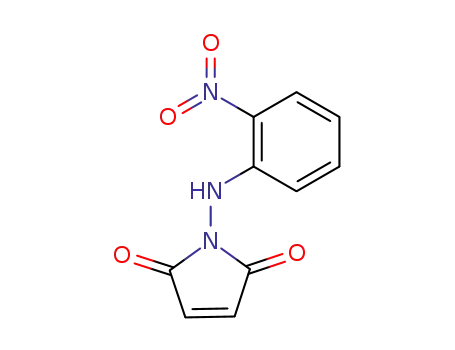
N-<2-Nitro-phenylamino>-maleinsaereimid
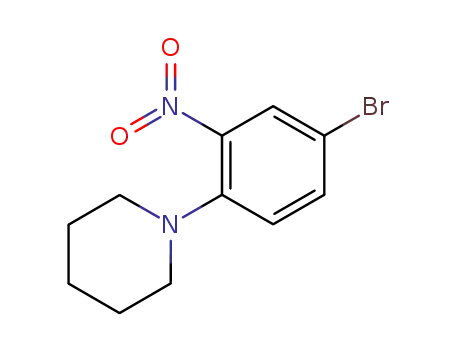
5-bromo-2-piperidin-1-yl-nitrobenzene
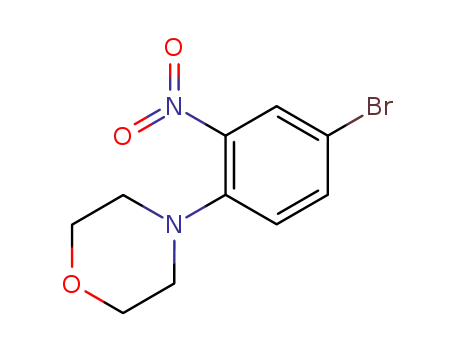
4-(4-bromo-2-nitro-phenyl)-morpholine
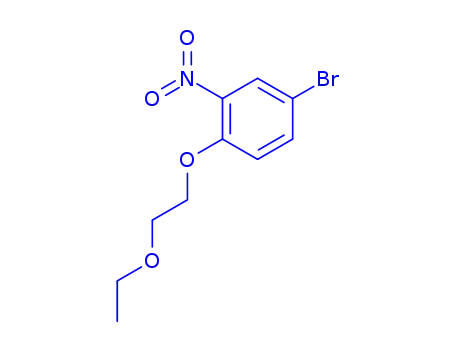
1-ethoxy-2-(4-bromo-2-nitro-phenoxy)-ethane
(5-bromo-2-methoxy-phenyl)-(4-bromo-2-nitro-phenyl)-amine
CAS:1746-13-0
CAS:14126-37-5
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97