Your Location:Home >Products >OLED intermediates >Thiophenes >119269-24-8


Product Details
Chemical Properties
Light yellow liquid
Uses
3-Hexadecylthiophene is used to prepare polythiophenes containing dithienopyrrole and benzothiadiazole with low band gap for red-emitting LEDs.
InChI:InChI=1/C20H36S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17-20-18-16-19-21-20/h16,18-19H,2-15,17H2,1H3
Conjugated self-assembled systems in water are of great interest because of their potential application in biocompatible supramolecular electronics, but so far their supramolecular chemistry remains almost unexplored. Here we present amphiphilic terthiophenes as a general self-assembling platform for the construction of conjugated aggregates, providing access to aggregates with different morphologies without changing the basic molecular design. We explored the design parameters of these amphiphilic terthiophenes in detail, leading to the selection and synthesis of in total 8 new amphiphilic oligothiophenes. Their aggregation behaviour was investigated by absorbance and fluorescence spectroscopy, dynamic light scattering, and cryo-transmission electron microscopy. Critical micelle concentrations as low as 0.01 mM were found and different sized aggregates ranging from several nanometres up to 200 nm. The aggregate morphology could also be tuned by changing the substitution pattern of hydrophilic and hydrophobic moieties, leading to different types of aggregates ranging from globular- and elongated micelles to bilayers. Remarkably, aggregation had only little effect on the electronic and spectroscopic properties of the oligothiophenes, which will be of interest for their application in supramolecular electronics.
Bolaamphiphilies with D-A-D type π-conjugated rigid cores composed by thiophenes as donors (D) and benzothiadiazole (BTD) as central acceptor (A) have been synthesised. Their self-assemblies and photophysical properties were investigated by polarising optical microscope, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. Such compounds can self-assemble into honeycomb cylinder mesophases with Colhex?/p6mm and Colsqu/p4mm lattices in their pure states as well as organogels with different morphologies in organic solvents. Their absorption spectra cover nearly the entire visible light range and their band gaps are relatively low. Tetrathiophene BTD based bolaamphilphiles (BT4/n) with higher D/A ratios than the bisthiophene BTD bolaamphilphiles (BT2/n) can self-assemble into more ordered nanostructures in both bulk states and solution. Both the absorption and emission peaks of BT4/n are strongly red shifted. The influence of the molecular conformation, the conjugated core length, as well as the D/A ratio on the self-assemble and photophysical characteristics of such D-A-D bolaamphiphiles are discussed.
A practical and convenient Co-catalyzed alkylation method for the facile introduction of various alkyl chains into organic electronically significant heteroaryl compounds, including thiophenes, furans, selenophenes, and pyrroles, is reported. Under well-optimized reaction conditions, a wide range of alkylated heteroaryl compounds have beeen efficiently prepared in moderate to good isolated yields. Notably, 2- or 3-alkylthiophenes, which play a decisive role in polymer chemistry and organic materials, have been synthesized step-economically for the first time by this reductive-coupling methodology using inexpensive cobalt salts as catalysts. This straightforward synthetic procedure avoids the preparation of moisture-unstable organometallic reagents (RMgX or RZnX) required in conventional alkylation protocols. Various alkyl chains have been introduced into organic, electronically important heteroaryl compounds step-economically through Co-catalyzed reductive alkylation reactions. The resulting alkylheteroarenes are indispensable building blocks for polymer chemistry and π-functional organic materials.
A series of 5,5′′′-diphenyl tetrathiophenes with polar glycerol groups at each end and two lateral flexible chains self-assemble into a series of liquid-crystalline honeycombs, formed by the π-conjugated rods which enclose polygonal prismatic cells filled by the lateral chains. With increasing chain length a discontinuous transition from triangular to square honeycombs takes place. At this transition a periodic honeycomb composed of a mixture of square and triangular cells in a ratio 1:2 was formed at low temperature, whereas at higher temperature a hexagonal columnar phase composed of triangular and randomly distributed rhombic cells, a new kind of cybotactic nematic phase, and also a cybotactic isotropic phase, both composed of square honeycomb fragments, represent the intermediate states. This provides an example of a dynamic self-assembled system where, depending on the molecular mobility, the transition between two periodic structures with different symmetry either leads to an increase of complexity, or to a chaotic regime with reduced order.
A series of 5,5′-diphenyl-2,2′-dithiophene based X-shaped polyphiles with two long lateral alkyl chains and terminal glycerol groups was synthesized and the liquid crystalline phases formed by these compounds were investigated by polarizing microscopy, DSC and XRD. These compounds form square (p4mm and p4gm) and hexagonal (p6mm) columnar LC phases. In these mesophases the molecules organize into polygonal honeycombs where the π-conjugated cores form the walls, fused at the edges by the hydrogen bonding networks between the glycerol units and filled by the lateral alkyl chains. By elongation of these chains, a series of polygonal honeycomb phases with a "single wall" structure, ranging from triangular via square and pentagonal to hexagonal was observed. Most triangular honeycombs appear to be defective and can be considered as mixtures of triangular cylinders with orientationally randomized rhombic cylinders. The transition from this improper triangular honeycomb to the square honeycomb takes place via a disordered isotropic phase. Addition of water to this isotropic phase gives rise to a true triangular honeycomb LC phase. Replacing one of the long lateral chains by a small methyl group leads to honeycombs formed by double walls instead of single walls. UV investigations indicate π-stacking of the aromatic cores organized in the honeycomb walls, which is of interest for the potential application of these materials in self assembled arrays of organic electronic material. This journal is

((E)-3-Hexadec-1-enyl)-thiophene


3-n-hexadecylthiophene
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
ethyl acetate;
under 2068.6 Torr;
|
72% |
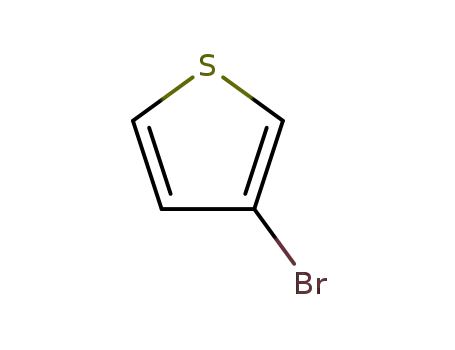
3-Bromothiophene

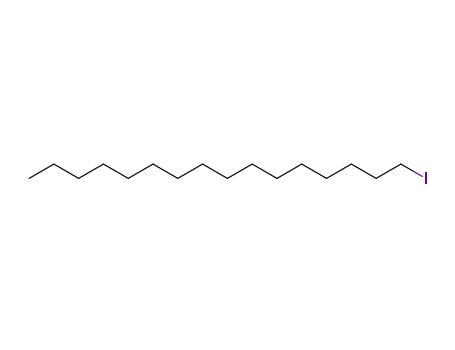
1-iodohexadecane


3-n-hexadecylthiophene
| Conditions | Yield |
|---|---|
|
With
pyridine; manganese; Tri(p-tolyl)phosphine; trifluoroacetic acid; cobalt(II) bromide;
In
N,N-dimethyl acetamide;
at 70 ℃;
for 24h;
Schlenk technique;
Inert atmosphere;
|
78% |

((E)-3-Hexadec-1-enyl)-thiophene

3-Bromothiophene

hexadecanyl bromide

pentadecyl bromide

2,5-dibromo-3-hexadecylthiophene
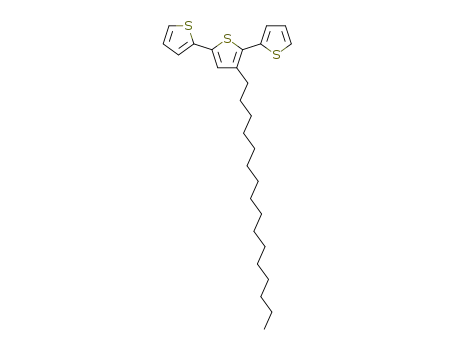
3-hexadecyl-2,2';5',2-terthiophene
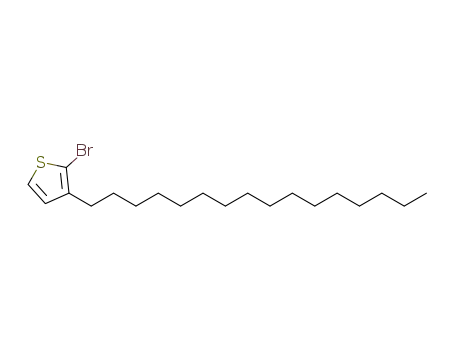
2-bromo-3-n-hexadecylthiophene
C66H94O6S4
CAS:855-38-9
CAS:110851-66-6
CAS:125143-53-5