Your Location:Home >Products >OLED intermediates >Thiophenes >110851-66-6


Product Details
Chemical Properties
Light yellow liquid
Uses
3-Tetradecylthiophene is a reactant used in the synthesis of 3''-alkyl-α-terthiophene derivatives with nematocidal activity.
InChI:InChI=1/C18H32S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-15-16-19-17-18/h15-17H,2-14H2,1H3
Aryl chlorides are better substrates than the corresponding bromides or iodides in the presented cross-coupling with alkyl Grignard reagents that is catalyzed by iron salts (see scheme); even aryl tosylates are converted efficiently. This situation is att
A series of X-shaped 5,5′-bis(phenylethynyl)-2,2′-bithiophene-based bolaamphiphiles bearing two long lateral alkyl chains and two terminal glycerol groups has been synthesized by using Kumada and Sonogashira coupling reactions as key steps. The thermotropic liquid crystalline behavior of these compounds was investigated by POM, DSC and X-ray scattering. With elongation of the lateral alkyl chains two different kinds of liquid crystalline phases with honeycomb structures, ColhexΔ/p6mm, formed by (defective) triangular honeycomb cells, and Colsqu/p4mm with square cells were observed for these compounds. UV and PL measurements indicate fluorescent properties making them potential candidates for application in fluorescence sensor devices.
A series of 5,5′-diphenyl-2,2′-dithiophene based X-shaped polyphiles with two long lateral alkyl chains and terminal glycerol groups was synthesized and the liquid crystalline phases formed by these compounds were investigated by polarizing microscopy, DSC and XRD. These compounds form square (p4mm and p4gm) and hexagonal (p6mm) columnar LC phases. In these mesophases the molecules organize into polygonal honeycombs where the π-conjugated cores form the walls, fused at the edges by the hydrogen bonding networks between the glycerol units and filled by the lateral alkyl chains. By elongation of these chains, a series of polygonal honeycomb phases with a "single wall" structure, ranging from triangular via square and pentagonal to hexagonal was observed. Most triangular honeycombs appear to be defective and can be considered as mixtures of triangular cylinders with orientationally randomized rhombic cylinders. The transition from this improper triangular honeycomb to the square honeycomb takes place via a disordered isotropic phase. Addition of water to this isotropic phase gives rise to a true triangular honeycomb LC phase. Replacing one of the long lateral chains by a small methyl group leads to honeycombs formed by double walls instead of single walls. UV investigations indicate π-stacking of the aromatic cores organized in the honeycomb walls, which is of interest for the potential application of these materials in self assembled arrays of organic electronic material. This journal is
A process for the production of compounds Ar—R1 by means of a cross-coupling reaction of an organometallic reagent R1—M with an aromatic or heteroaromatic substrate Ar—X catalyzed by one or several iron salts or iron complexes as catalysts or pre-catalysts, present homogeneously or heterogeneously in the reaction mixture. This new invention exhibits substantial advantages over established cross coupling methodology using palladium- or nickel complexes as the catalysts. Most notable aspects are the fact that (i) expensive and/or toxic nobel metal catalysts are replaced by cheap, stable, commercially available and toxicologically benign iron salts or iron complexes as the catalysts or pre-catalysts, (ii) commercially attractive aryl chlorides as well as various aryl sulfonates can be used as starting materials, (iii) the reaction can be performed under “ligand-free” conditons, and (iv) the reaction times are usually very short.

((E)-3-Tetradec-1-enyl)-thiophene


3-n-tetradecylthiophene
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
ethyl acetate;
under 2068.6 Torr;
|
86% |
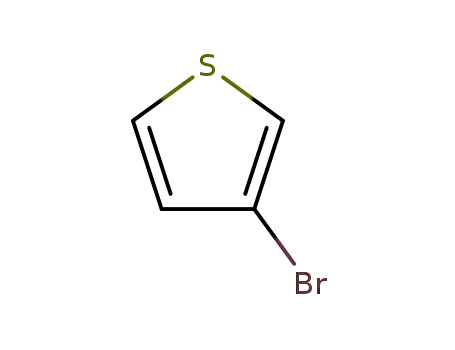
3-Bromothiophene


tetradecylmagnesium bromide


3-n-tetradecylthiophene
| Conditions | Yield |
|---|---|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
for 15h;
Reflux;
|
80% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
for 15h;
Reflux;
|

((E)-3-Tetradec-1-enyl)-thiophene
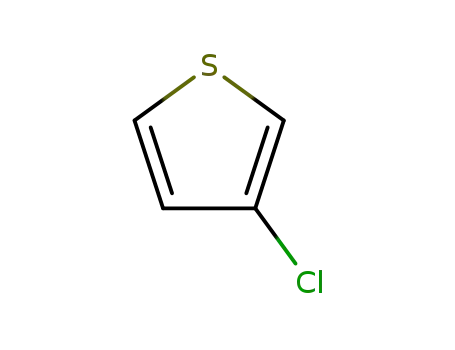
3-chlorothiophene

tetradecylmagnesium bromide

1-bromotridecane
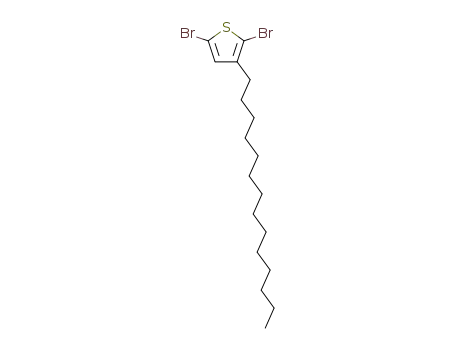
2,5-dibromo-3-tetradecylthiophene
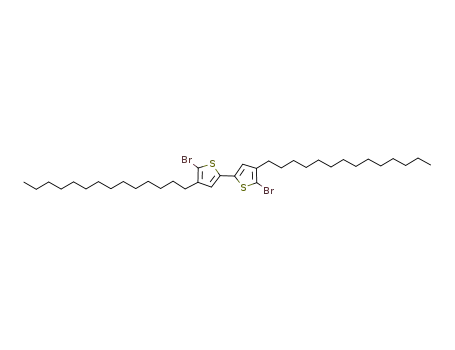
2,2'-dibromo-3,3'-bis(n-tetradecyl)-5,5'-bithiophene

2-bromo-3-n-tetradecylthiophene
CAS:1016-05-3
CAS:104934-52-3
CAS:119269-24-8