Your Location:Home >Products >OLED intermediates >Thiophenes >52431-30-8
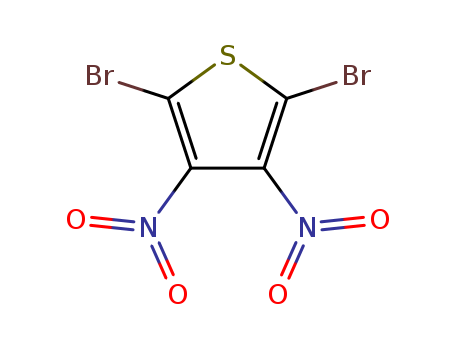

Product Details
Chemical Properties
Light Orange Solid
Uses
Reatant in the synthesis of bromodinitro compound, polymers via stille coupling. 3,4-diaminothiophene was obtained from the reduction of 2,5-dibromo-3,4-dinitrothiophene.
Uses
This monomer is useful in the Pd catalyzed Stille coupling to make conjugated thiophene oligomers or co-oligomers. The corresponding amine(s) can be obtained by subsequent reduction.
InChI:InChI=1/C4Br2N2O4S/c5-3-1(7(9)10)2(8(11)12)4(6)13-3
(Chemical Equation Presented) (Graph Presented) The synthesis and the optical and electrochemical properties of thiophene end capped oligo(2,3-alkylthieno[3,4-b]pyrazine)s are presented. The optical absorption rapidly shifts to lower energies with increasing chain length, caused in almost equal amounts by a rise of the HOMO and a lowering of the LUMO levels. The optical band gap of the polymer is estimated to be 1.13 ± 0.07 eV. Extrapolated redox potentials indicate that the polymer is a small band gap p-type material.
New classes of thieno[3,4-b]pyrazines containing thienyl and ethylenedioxy phenyl units on electron-withdrawing moieties of π-conjugated terthienyl were synthesized. The effect of structural differences on electrochemical and optoelectronic properties of the resulting polymers was investigated. Changes in the electronic nature of the functional groups enable to tune the electrochemical properties of the π-conjugated terthienyl monomers by lowering oxidation potential from 0.62 V (DTTP) to 0.56 V (DBTP). Spectroelectrochemical analyses revealed that the neutral polymer (PDBTP) is dark green in its neutral state revealing π-π* transitions in two well-separated bands at 410 and 751 nm. The electronic band gap of polymer, defined as the onset of the π-π* transition, is found to be 1.0 eV. Using the thienyl unit instead of ethylenedioxy phenyl, a red shift in the band gap (0.95 eV) is observed. The polymer, PDTTP, exhibits multicolor electrochromism and can be switched between a dark yellow neutral state, a green intermediate state, and a brown oxidized state. PDBTP also shows a multicolored electrochromic behavior with three distinct states: dark green at the neutral state, a brown intermediate state, and a brown-violet oxidized state.
A series of thienopyrazine-based donor-acceptor-donor (D-A-D) near-infrared (NIR) fluorescent compounds were synthesized through a rapid, palladium-catalyzed C-H activation route. The dyes were studied through computational analysis, electrochemical prope
The invention provides a nitration method of a simple and efficient aromatic heterocyclic compound, which is large in width, easy to operate and good in repeatability. By means of the method, high-efficiency nitration of common aromatic compounds is realized, the nitration efficiency is greatly improved, and the simple synthesis of the organic photoelectric material intermediate is realized.
Organic Near-Infrared luminophores have found broad application as functional materials, but the development of efficient NIR emitters is still a challenging goal. Here we report on a new class of thieno[3,4-b]pyrazine-based NIR emitting materials with Aggregation Induced Emission (AIE) properties. The dyes feature a donor–acceptor–donor (D?A?D) structure, with a thienopyrazine acceptor core connected to two triarylamine donor groups bearing a tetraphenylethylene (TPE) moiety. Fast and efficient synthesis allowed the modular preparation of three dyes of tunable absorption and emission profiles. These constructs were extensively characterized by spectroscopic studies in different solvents, which revealed intense light-harvesting ability and emissions in the deep-red and NIR region with large Stokes shift values. Remarkably, the dyes exhibited AIE properties, retaining emissive ability in the aggregate state, thus emerging as attractive materials for their potential application in the development of luminescent devices.
Current approaches to synthesize π-conjugated polymers (CPs) are dominated by thermally driven, transition-metal-mediated reactions. Herein we show that electron-deficient Grignard monomers readily polymerize under visible-light irradiation at room temperature in the absence of a catalyst. The product distribution can be tuned by the wavelength of irradiation based on the absorption of the polymer. Conversion studies are consistent with an uncontrolled chain-growth process; correspondingly, chain extension produces all-conjugated n-type block copolymers. Preliminary results demonstrate that the polymerization can be expanded to donor–acceptor alternating copolymers. We anticipate that this method can serve as a platform to access new architectures of n-type CPs without the need for transition-metal catalysis.
The present invention relates to a triphenylamine compound, to a polymer thereof and to an electrochromic device comprising the same, and specifically, to a triphenylamine compound represented by the chemical formula (1), to a polymer thereof and to an electrochromic device comprising the same. In the chemical formula (1), R, R1, R2, R3, R4, and R5 are as defined in claim 1. The present invention also provides a triphenylamine-based polymer capable of forming a thin film having uniform surface and exhibiting excellent reversibility in the electrochromic characteristics.COPYRIGHT KIPO 2018
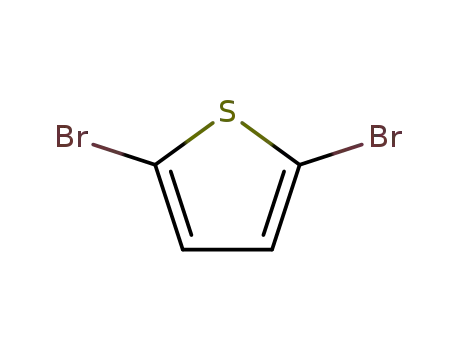
2,5-dibromothiophen

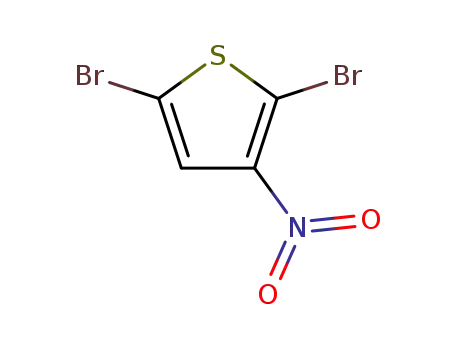
2,5-dibromo-3-nitro-thiophene

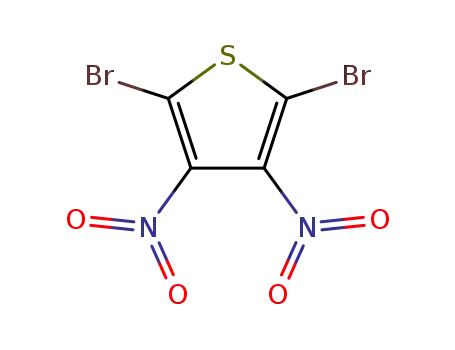
2,5-dibromo-3,4-dinitrothiophene
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; nitric acid;
|
2,5-dibromothiophen


2,5-dibromo-3,4-dinitrothiophene
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; nitric acid;
for 3h;
|
94% |
|
With
sulfuric acid; sulfur trioxide; nitric acid;
at 30 ℃;
for 3h;
|
94% |
|
With
sulfuric acid; nitric acid;
In
water;
at 20 - 30 ℃;
for 4h;
Cooling with ice;
|
88% |
|
With
sulfuric acid; nitric acid;
at 15 - 30 ℃;
Cooling with ice;
|
81% |
|
With
trifluorormethanesulfonic acid; sulfuric acid; nitric acid;
at 30 ℃;
for 21.75h;
Cooling with ice;
|
70% |
|
With
sulfuric acid; nitric acid;
|
67% |
|
With
sulfuric acid; nitric acid;
|
67% |
|
With
sulfuric acid; nitric acid;
at 20 - 30 ℃;
for 3h;
|
66% |
|
With
sulfuric acid; nitric acid;
at 0 - 20 ℃;
for 3h;
|
55% |
|
With
sulfuric acid; nitric acid;
at 15 ℃;
for 4h;
Heating;
|
54% |
|
With
sulfuric acid; nitric acid;
at 30 ℃;
Inert atmosphere;
|
52% |
|
With
sulfuric acid; nitric acid;
at 0 - 15 ℃;
for 3h;
|
52% |
|
With
sulfuric acid; nitric acid;
at 20 - 30 ℃;
for 3h;
|
48% |
|
With
sulfuric acid; nitric acid;
at 20 - 30 ℃;
for 3h;
|
47% |
|
With
sulfuric acid; cis-nitrous acid;
at 20 ℃;
Cooling with ice;
|
43.7% |
|
With
sulfuric acid; nitric acid;
at 0 ℃;
for 1h;
Inert atmosphere;
|
40% |
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
at 20 - 30 ℃;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
at 20 ℃;
for 2h;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
2,5-dibromothiophen
2,3,5-tribromothiophene
thiophene
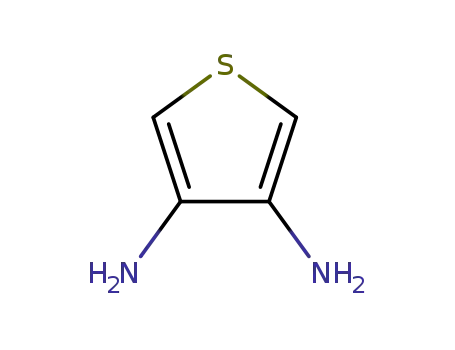
thiophene-3,4-diamine
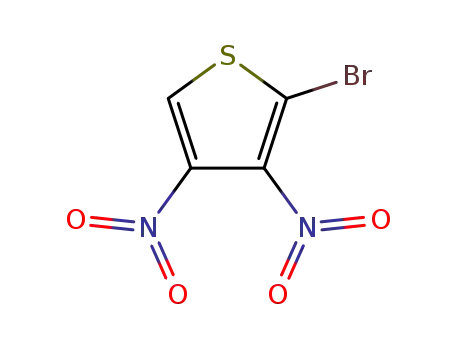
2-bromo-3,4-dinitrothiophene
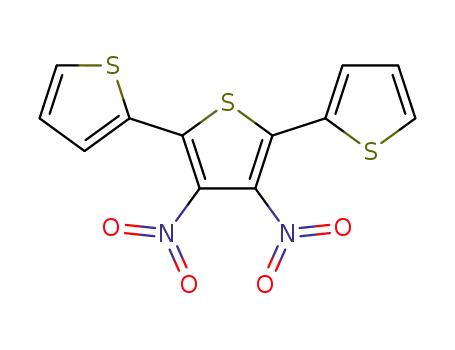
3’,4’-dinitro-2,2’:5’,2’’-terthiophene
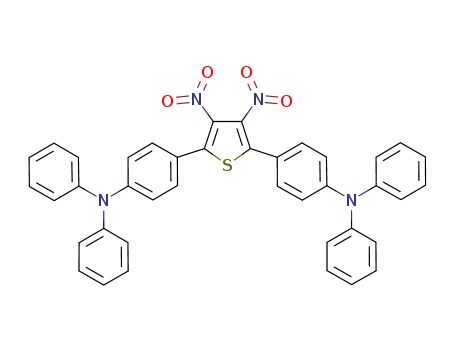
4,4'-(3,4-dinitrothiophene-2,5-diyl)bis(N,N-diphenylaniline)
CAS:1770840-43-1
Molecular Formula:C6H5IN4
Molecular Weight:260.04
CAS:867374-53-6
CAS:3140-93-0
CAS:14282-76-9