Your Location:Home >Products >Organic phosphines >Phenyl phosphines >27721-02-4
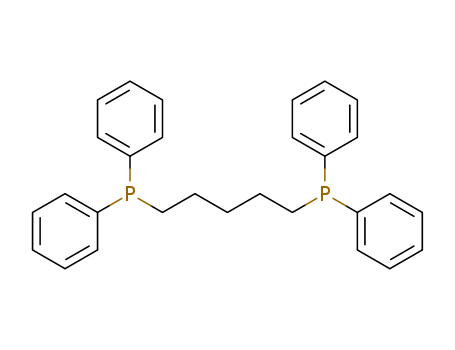

Product Details
Chemical Properties
white to light yellow crystal powde
Uses
1,5-Bis(diphenylphosphino)pentane is used as a ligand for the cross coupling reactions. It is used in the manufacturing of glass bottle PTFE bottle, aluminum foil bag and in cardboard drum. combination of a 1,5-bis(diphenylphosphino)pentane ligand and the addition of N,N,N?,N?-tetramethylethylenediamine (TMEDA) as a co-catalyst are the key factors in obtaining the corresponding aryl nitriles with improved catalyst productivities and selectivities.
InChI:InChI=1/C29H30P2/c1-6-16-26(17-7-1)30(27-18-8-2-9-19-27)24-14-5-15-25-31(28-20-10-3-11-21-28)29-22-12-4-13-23-29/h1-4,6-13,16-23H,5,14-15,24-25H2
Optimal parameters of organomagnesium technique of synthesis of triethylgallium have been defined. Various techniques of deep purification of triethylgallium to the extent required in metalorganic vapor-phase epitaxy MOVPE have been studied: by way of residue ether displacement through high-performance rectification and interaction with high pure aluminum and gallium trichloride, and by way of reversible complexation with triphenylphosphine, 1,3-bis(diphenylphosphine)propane and 1,5- bis(diphenylphosphine)pentane. Advantages and disadvantages of each technique have been identified. We have shown high performance of adduct purification technique covering trimethyl and triethyl derivatives of aluminum, gallium and indium. The structure of donor-acceptor complexes between metal alkyls and the above-mentioned phosphines have been verified using H and 31P NMR spectroscopy and X-ray studies, as well as quantum chemical calculations. Thermal stability of triethylgallium and oxidation of its adducts with phosphines have been studied.
A mild and efficient method for the synthesis of ditertiary phosphines has been developed. In the presence of cesium hydroxide, molecular sieves, and DMF, various dihalides were coupled with diphenylphosphine at room temperature, and the results have demonstrated that this methodology offers a general synthetic procedure producing a variety of ditertiary phosphines in high yields.
A mild and efficient method for the synthesis of tertiary phosphines and ditertiary phosphines has been developed. In the presence of cesium hydroxide, molecular sieves and DMF at room temperature, various secondary phosphines and alkyl bromides were examined, and the results have demonstrated that this methodology offers a general synthetic procedure to produce tertiary phosphines in moderate to high yields. Optically active tertiary phosphine synthesis is also described.
When oganohalosilanes are prepared by charging a reactor with a contact mass containing a metallic silicon powder and a copper catalyst, and introducing an organohalide-containing gas into the reactor to effect the direct reaction, a poly(organo)phosphino compound is added to the contact mass. The invention is successful in producing organohalosilanes at a significantly improved production rate without reducing the selectivity of useful silane.
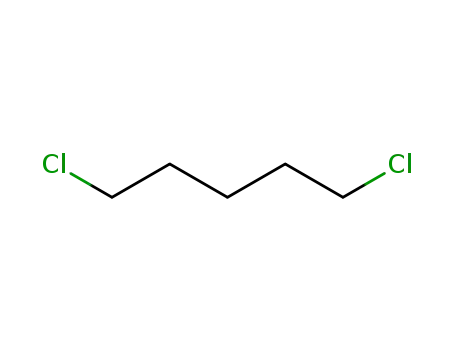
1,5-dichloropentane


lithium

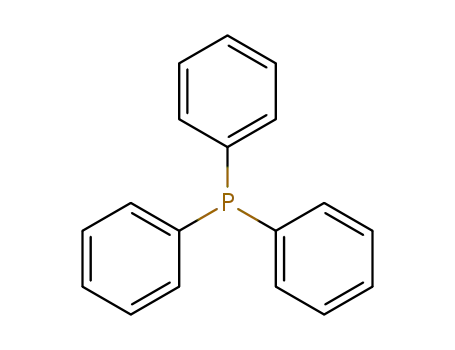
triphenylphosphine

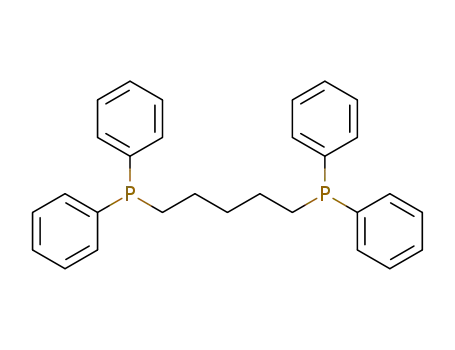
1,5-bis-(diphenylphosphino)pentane
| Conditions | Yield |
|---|---|
|
lithium; triphenylphosphine;
In
tetrahydrofuran;
at 50 ℃;
Inert atmosphere;
1,5-dichloropentane;
In
tetrahydrofuran;
at 0 - 80 ℃;
Inert atmosphere;
|
35% |
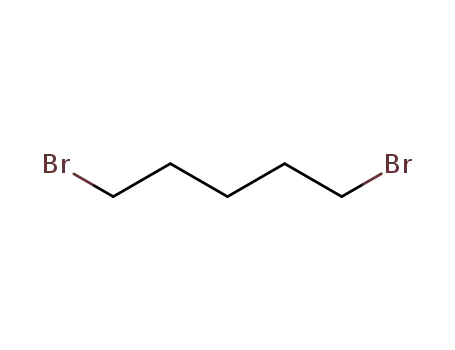
1,5-dibromo-pentane

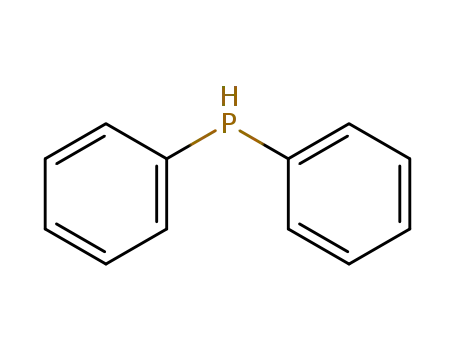
diphenylphosphane


1,5-bis-(diphenylphosphino)pentane
| Conditions | Yield |
|---|---|
|
With
cesium hydroxide; 4 Angstroem MS;
In
N,N-dimethyl-formamide;
at 23 ℃;
for 72h;
|
63% |
|
With
cesium hydroxide; 4 A molecular sieve;
In
N,N-dimethyl-formamide;
at 23 ℃;
for 72h;
|
63% |

1,5-dibromo-pentane

diphenylphosphane

1,5-dichloropentane

lithium
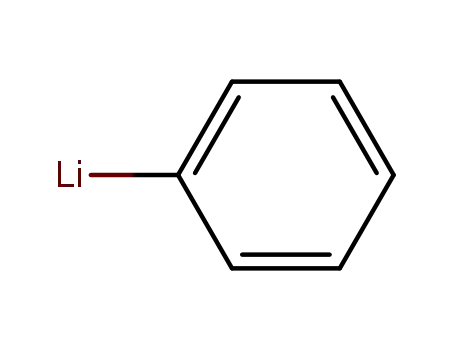
phenyllithium
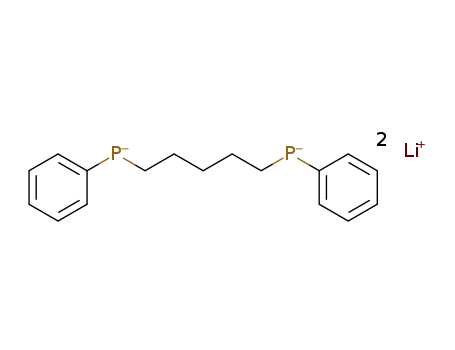
1,5-Bis-
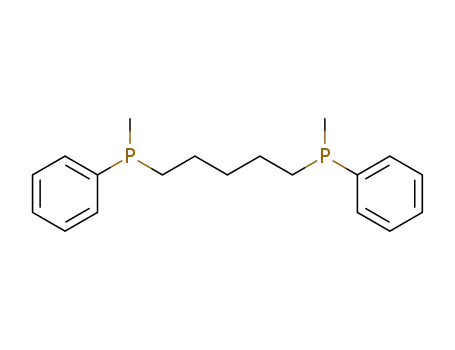
1,5-di(methyl-phenyl-phosphino)pentane
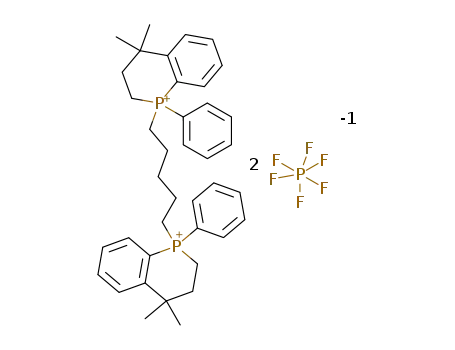
1,1'-(1,5-pentanediyl)bis(1,2,3,4-tetrahydro-4,4-dimethyl-1-phenylphosphinolinium bis
CAS:13716-12-6
Molecular Formula:C<sub>12</sub>H<sub>27</sub>P
Molecular Weight:202.32
CAS:19845-69-3