Your Location:Home >Products >OLED intermediates >Carbazoles >10537-08-3
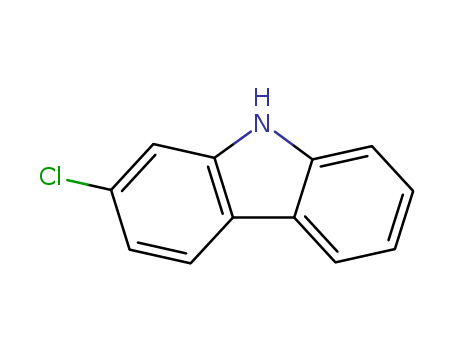

Product Details
Uses
2-Chloro-9H-carbazole is a useful research chemical.
Visible-light-driven photochemical Cadogan-type cyclization has been discovered. The organic D-A type photosensitizer 4CzIPN found to be an efficient mediator to transfer energy from photons to the transient intermediate that breaks the barriers of deoxygenation in Cadogan reaction and enables a mild metal-free access to carbazoles and related heterocycles. DFT calculation results indicate mildly endergonic formation of the intermediate complex of nitrobiarenes and PPh3, which corresponds with experimental findings regarding reaction temperature. The robust synthetic capacity of the photoredox Cadogan reaction systems has been demonstrated by the viable productivity of a broad range of carbazoles and related N-heterocycles with good tolerance of various functionalities.
A mild transition-metal- and photosensitizer-free photoredox system based on the combination of NaI and PPh3 was found to enable highly selective reduction of nitroarenes. This protocol tolerates a broad range of reducible functional groups such as halogen (Cl, Br, and even I), aldehyde, ketone, carboxyl, and cyano. Moreover, the photoredox catalysis with NaI and stoichiometric PPh3 provides also an alternative entry to Cadogan-type reductive amination when o-nitrobiarenes were used.
While direct nitrene insertions into C?H bonds have become an important tool for building C?N bonds in modern organic chemistry, the generation of nitrene intermediates always requires transition metals, high temperatures, ultraviolet or laser light. We report a mild synthesis of carbazoles and related building blocks through a visible light-induced intramolecular C?H amination reaction. A striking advantage of this new method is the use of more reactive aryl sulfilimines instead of the corresponding hazardous azides. Different catalysts and divergent light sources were tested. The reaction scope is broad and the product yield is generally high. An efficient gram-scale synthesis of Clausine C demonstrates the applicability and scalability of this new method.
The present invention belongs to the field of organic chemistry, and particularly relates to a preparation method for 2-substituted carbazole compounds. According to the preparation method provided by the present invention, the raw material source is wide, reaction operation and post-treatment are simple and convenient, the yield is high, the application range is wide, and the industrial production is facilitated.
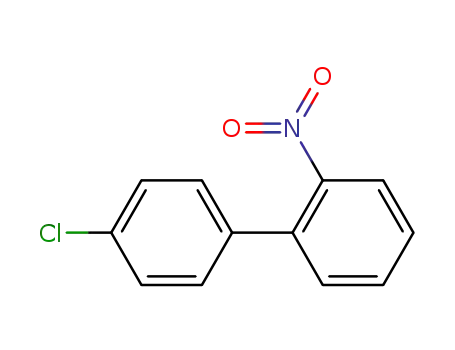
4'-chloro-2-nitrobiphenyl

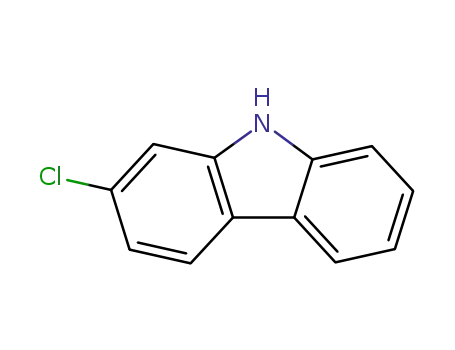
2-chloro-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
(4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; triphenylphosphine;
In
1,4-dioxane;
at 55 ℃;
for 72h;
Inert atmosphere;
Irradiation;
|
89% |
|
With
triethyl phosphite;
In
N,N-dimethyl-formamide;
at 80 - 150 ℃;
Inert atmosphere;
|
85% |
|
With
triethyl phosphite;
at 210 ℃;
for 0.166667h;
microwave irradiation;
|
74% |
|
With
triphenylphosphine; sodium iodide;
In
1,4-dioxane;
at 60 ℃;
for 72h;
Inert atmosphere;
Irradiation;
|
64% |
|
With
triethyl phosphite;
In
nitrogen;
at 150 ℃;
for 24h;
Inert atmosphere;
|
60% |
|
With
phenylmagnesium bromide;
In
tetrahydrofuran;
at 0 ℃;
for 0.25h;
Inert atmosphere;
|
58% |
|
With
triethyl phosphite;
|
|
|
Multi-step reaction with 2 steps
1: acetonitrile / 20 °C / Glovebox; Inert atmosphere
2: 72 h / 120 °C / Inert atmosphere
In
acetonitrile;
|
![4’-chloro-[1,1’-biphenyl]-2,2’-iodonium triflate](/upload/2023/2/7ed75124-b482-421f-8c09-c74e6e2ad6d1.png)
4’-chloro-[1,1’-biphenyl]-2,2’-iodonium triflate


2-chloro-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
sodium azide; copper(I) thiophene-2-carboxylate; sodium hydrogencarbonate; triphenylphosphine;
In
N,N-dimethyl acetamide;
at 100 ℃;
for 12h;
Inert atmosphere;
Schlenk technique;
|
74% |
|
With
sodium azide; copper(I) thiophene-2-carboxylate; sodium hydrogencarbonate; triphenylphosphine;
In
N,N-dimethyl acetamide;
at 100 ℃;
for 12h;
Inert atmosphere;
Schlenk technique;
|
74% |
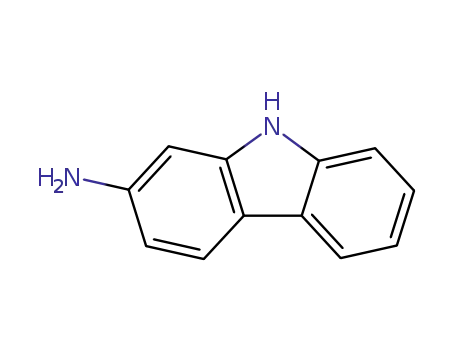
2-aminocarbazole
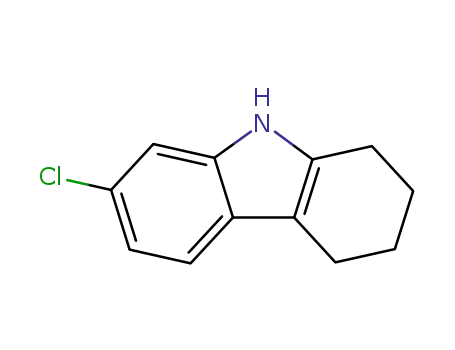
7-chloro-2,3,4,9-tetrahydro-1H-carbazole
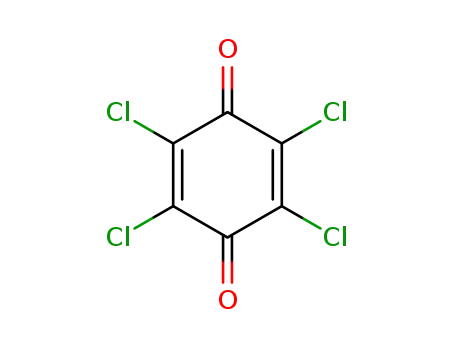
chloranil
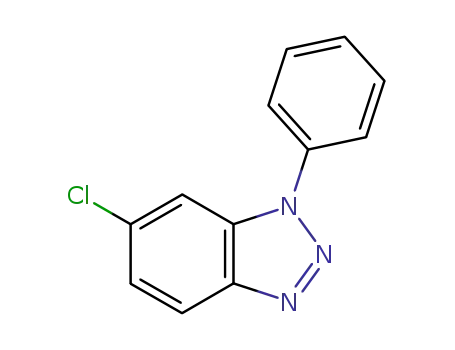
6-chloro-1-phenyl-1H-benzotriazole
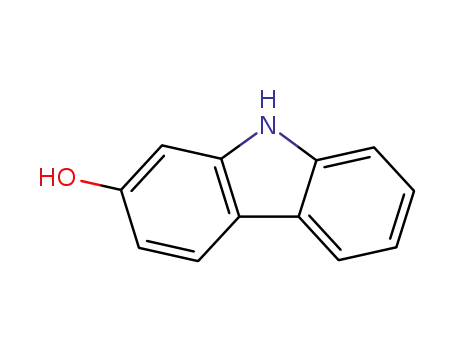
2-hydroxycarbazole
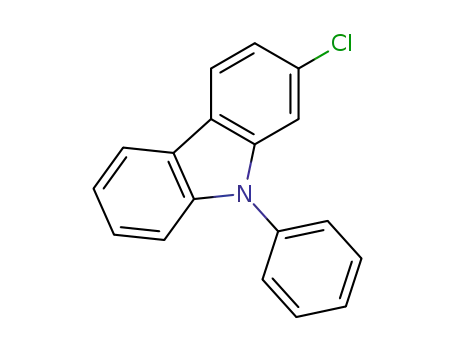
2-chloro-9-phenylcarbazole
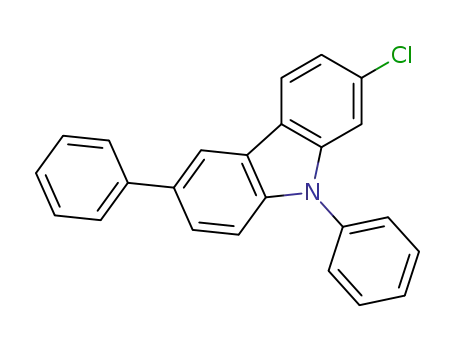
2-chloro-6-phenyl-N-phenylcarbazole
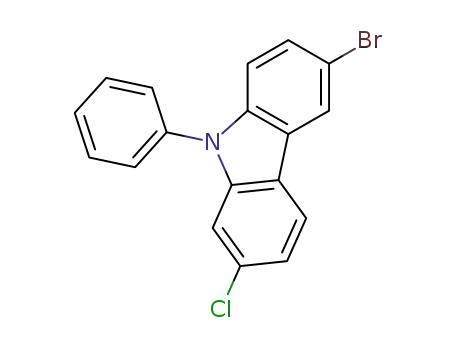
2-chloro-6-bromo-N-phenylylcarbazole
CAS:65016-62-8
CAS:2489-86-3