Your Location:Home >Products >OLED intermediates >Fluorenes >462128-39-8
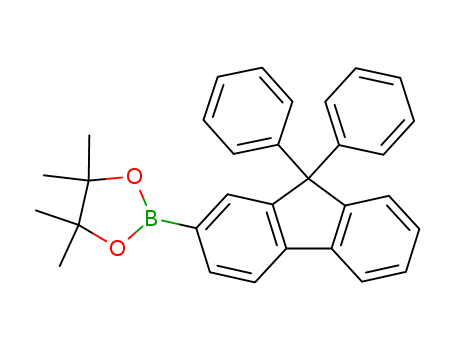

Product Details
(Matrix Presented) A series of novel 9,9-diarylfluorene-capped oligothiophenes were synthesized by Suzuki coupling reactions in good yields. The color of the emissions can be controlled by varying the conjugation length of the oligothiophene core. The bulky and rigid terminal groups of the resulting oligomers are significantly beneficial for their high morphological and thermal stability. These new oligothiophenes exhibit intriguing reversible oxidation and reduction redox behavior.
The invention provides an organic compound and an electronic element and an electronic device thereof, and belongs to the technical field of organic electroluminescence. The organic compound has a structural formula shown 1, wherein the organic compound h
The invention relates to an organic compound with high mobility, and application thereof. The organic compound provided by the invention takes a benzene ring connected with a five-membered parallel ring structure as a core, and has good thermal stability, high glass transition temperature and appropriate HOMO energy level. A device adopting the organic compound provided by the invention can effectively improve the photoelectric property of an OLED device and prolong the service life of the OLED device through structural optimization.
The invention provides a compound for fabricating an electron transport layer. The compound has a molecular formula as described in the specification. The invention also provides an organic light emitting diode and an organic light emitting diode display
The present invention relates to a compound for an organic electronic element and an organic electronic element using the same. To the present invention, an organic electronic element having high luminous efficiency, low driving voltage, and high heat resistance can be provided and the color purity and lifetime of the organic electronic element can be improved. (by machine translation)
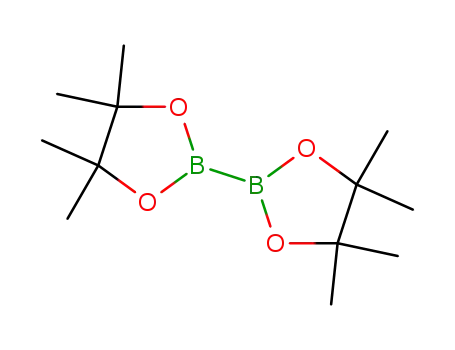
bis(pinacol)diborane


2-bromo-9,9-diphenyl-9H-fluorene

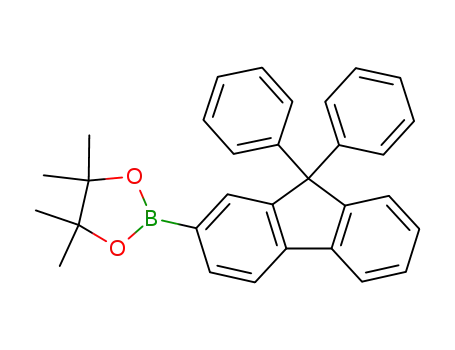
2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-diphenyl-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
N,N-dimethyl-formamide;
Reflux;
|
91% |
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
at 85 ℃;
for 8h;
Inert atmosphere;
|
76% |
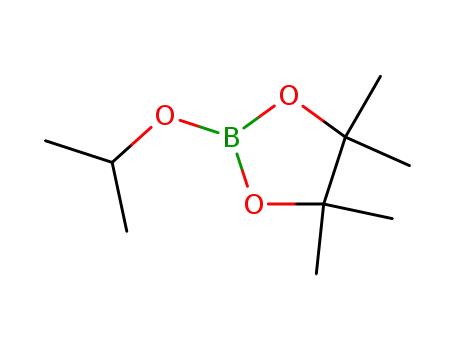
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane


2-bromo-9,9-diphenyl-9H-fluorene


2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-diphenyl-9H-fluorene
| Conditions | Yield |
|---|---|
|
2-bromo-9,9-diphenyl-9H-fluorene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
Further stages.;
|
82% |

2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

2-bromo-9,9-diphenyl-9H-fluorene

bis(pinacol)diborane

C31H21I
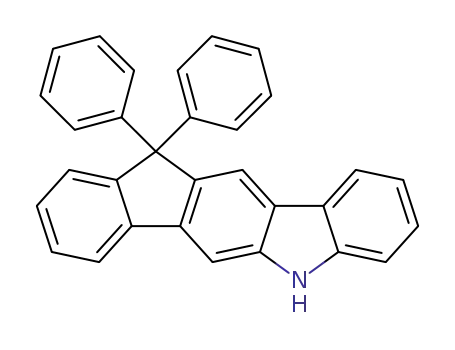
C31H21N
CAS:1346669-48-4
Molecular Formula:C36H24N2
Molecular Weight:484.6
CAS:100953-52-4
CAS:1224976-40-2
CAS:34172-50-4