Your Location:Home >Products >OLED intermediates >Fluorenes >1224976-40-2
Product Details
The present specification provides an anthracene-based compound of chemical formula 1, and an organic light emitting device having the same. By having a substituent including an aryl group or a heteroaryl group at a position of 9 and 10 of anthracene, and applying the substituent to the organic light emitting device, the light emitting efficiency and durability of the organic light emitting device can be significantly improved.COPYRIGHT KIPO 2018
The present invention describes carbazole, dibenzofuran, dibenzothiophene and fluorene derivatives which are substituted by electron-deficient heteroaryl groups, in particular for use as triplet matrix materials in organic electroluminescent devices. The invention furthermore relates to a process for the preparation of the compounds according to the invention and to electronic devices comprising these compounds.
The invention provides an anthracene-type organic electroluminescent compound represented as the structure formula I, wherein the compound is excellent in thermal stability, is high in luminescent efficiency and luminescent purity, and can be used for producing OLEDs in the fields of organic solar cells, organic thin-film transistors or organic photoreceptors. The invention also provides the OLED including a positive pole, a negative pole and an organic layer. The organic layer is composed of one or more than one from a luminescent layer, a hole injection layer, a hole transport layer, an exciton barrier layer, an electron injection layer and an electron transport layer. At least one layer of the organic layer includes the compound represented as the structure formula I.
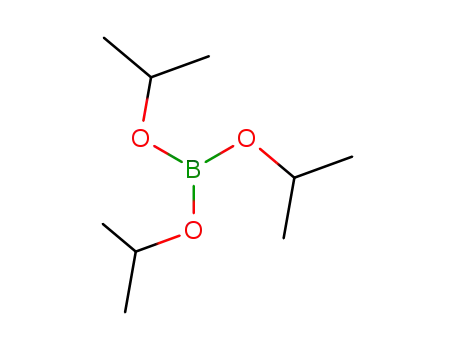
Triisopropyl borate

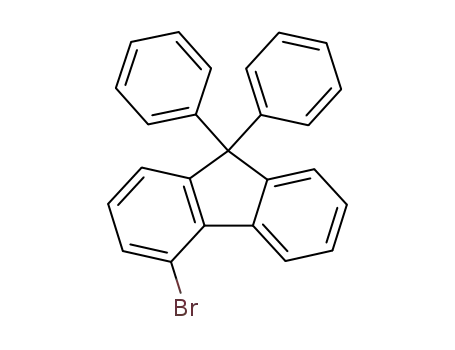
4-bromo-9,9-diphenyl-9H-fluorene

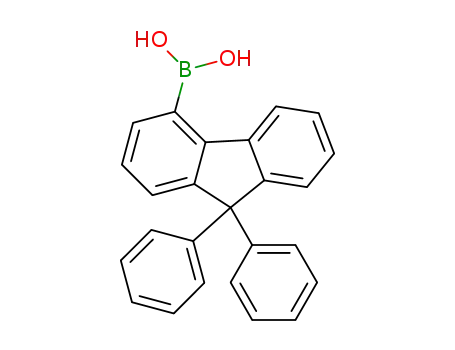
(9,9-diphenyl-9H-fluoren-4-yl)boronic acid
| Conditions | Yield |
|---|---|
|
4-bromo-9,9-diphenyl-9H-fluorene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
|
76% |


(9,9-diphenyl-9H-fluoren-4-yl)boronic acid
| Conditions | Yield |
|---|---|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 5h;
With
hydrogenchloride;
In
tetrahydrofuran;
|
88% |
|
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2.5h;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 18h;
|
78% |

Triisopropyl borate

4-bromo-9,9-diphenyl-9H-fluorene
CAS:6002-34-2
CAS:1259280-37-9
CAS:462128-39-8