Your Location:Home >Products >OLED intermediates >Fluorenes >159-62-6
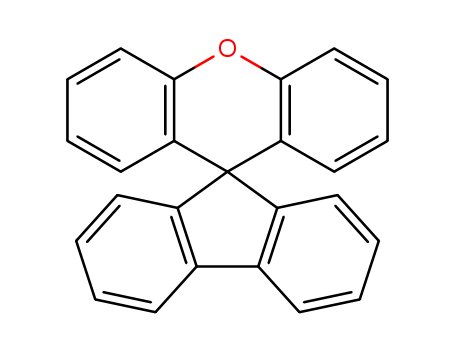

Product Details
Four heterocyclic spiro-type hole transporting materials (HTMs) carrying a spiro[fluorene-9,9′-xanthene] (SFX) (SFX-TPAM and SFX-TPA) or spiro[fluorene-9,9′-thioxanthene] (SFT) unit (SFT-TPAM and SFT-TPA) were synthesized through a low-cost facile route with high yields for perovskite solar cell (PSC) applications. In terms of absorption, these four compounds in the film state are all transparent at wavelengths longer than 430 nm, which is beneficial for allowing visible light to reach the perovskite active layer without being absorbed by the hole transporting layer (HTL). The photovoltaic performance of the inverted PSCs based on these small molecular HTMs with the device architecture of glass/ITO/HTL/CH3NH3PbI3/C60/BCP/Ag was tested. Only SFX-TPAM had its highest occupied molecular orbital (HOMO) level matched with the valence band of CH3NH3PbI3. The inverted PSC based on a dopant-free SFX-TPAM HTL achieves a power conversion efficiency of 10.23% under the illumination of standard one sun lighting, which is better than that (8.17%) of the cell based on dopant-free spiro-OMeTAD. The better photovoltaic performance of SFX-TPAM compared to spiro-OMeTAD may be due to the MAPbI3 film deposited on it having better quality. These results indicate that the facilely synthesized, low-cost SFX based small molecules can be used as the HTMs for PSCs.
A universal thermally activated delayed fluorescence (TADF) host, 4′-diphenylphosphinoylspiro[fluorene-9,9′-xanthene] (SFXSPO), is constructed with a highly distorted and asymmetric configuration and disordered molecular packing in its solid state. SFXSPO
An unexpected one-pot synthetic approach toward spiro[fluorene-9,9′- xanthene] (SFX) under excessive MeSO3H conditions has been developed. The key step involves a thermodynamically controlled cyclization reaction. Blue-light-emitting materials based on SFX building blocks that exhibit high thermal stability have also been synthesized.
The invention provides a method for synthesizing spirofluorene xanthene based on a microreactor, namely, in a microreactor, a proportional fluorenone derivative and a phenolic derivative are introduced, and under acid catalysis 140 - 180 °C reaction 5 - 410 minutes, through F-C cyclization reaction, the solvent is screened through screening. A multi-substituted or multi-functionalized spirofluorene xanthene aromatic hydrocarbon is synthesized by regulating the flow rate, regulating the mole ratio and the like in one step. Compared with the traditional method, the method provided by the invention greatly shortens the reaction time, shortens the reaction time from 24 hours to 46 minutes, improves the production efficiency, and improves the yield of spirofluorene xanthene to 72%. The method has the advantages of accessible raw materials, simple operation, high yield, atomic economy and the like, and is not only suitable for continuous synthesis in laboratories, but also capable of easily realizing industrial macro preparation.
Four spiro[fluorene-9,9′-xanthene] (SFX) derivatives, SFX-TAD, SFX-TCz, SFX-TPTZ and SFX-MeOTAD have been synthesized for use as hole-transport materials and fully characterized by 1H/13C NMR spectroscopy, mass spectrometry, XRD and
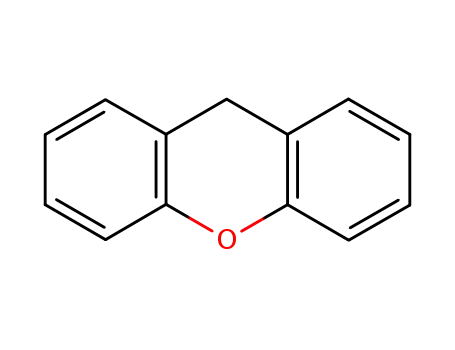
xanthene

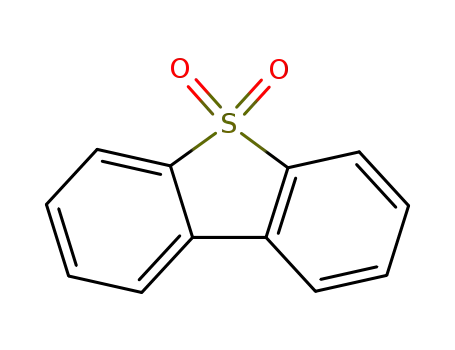
dibenzothiophene sulfone

![2'-(9H-xanthen-9-yl)-[1,1'-biphenyl]-2-sulfinic acid](/upload/2023/2/70aa42ec-dc70-493a-83df-4f917961cc2c.png)
2'-(9H-xanthen-9-yl)-[1,1'-biphenyl]-2-sulfinic acid

![spiro[fluorene-9,9’-xanthene]](/upload/2023/2/a389895c-0204-4225-a196-cdbc083d7c24.png)
spiro[fluorene-9,9’-xanthene]
| Conditions | Yield |
|---|---|
|
With
potassium hexamethylsilazane;
In
1,4-dioxane; toluene;
at 80 ℃;
for 1.5h;
Inert atmosphere;
|
60% 39% |
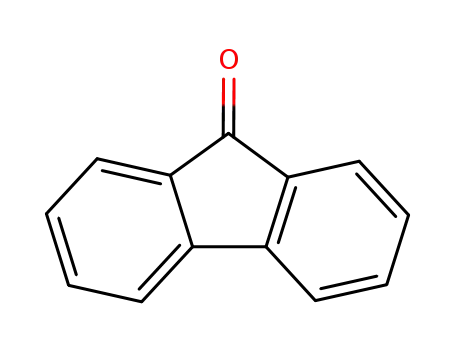
9-fluorenone

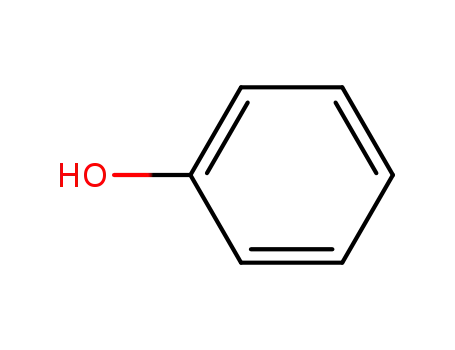
phenol

![spiro[fluorene-9,9’-xanthene]](/upload/2023/2/a389895c-0204-4225-a196-cdbc083d7c24.png)
spiro[fluorene-9,9’-xanthene]
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid;
at 150 ℃;
for 24h;
|
80% |
|
With
methanesulfonic acid;
at 150 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
77% |
|
With
methanesulfonic acid;
In
neat (no solvent);
at 150 ℃;
for 24h;
|
75% |
|
With
methanesulfonic acid;
In
1,2-dichloro-benzene;
at 150 ℃;
for 0.666667h;
Flow reactor;
Industrial scale;
|
72% |
|
With
trifluorormethanesulfonic acid;
at 80 ℃;
|
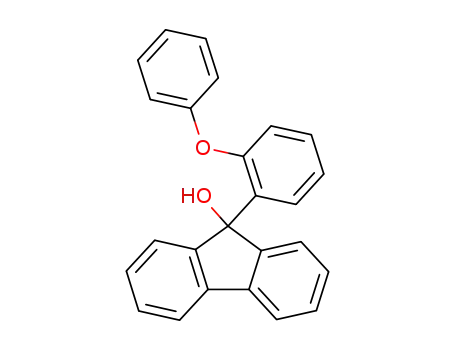
9-hydroxy-9-(2-phenoxy-phenyl)-fluorene
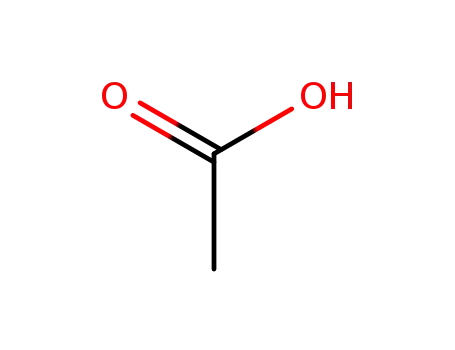
acetic acid
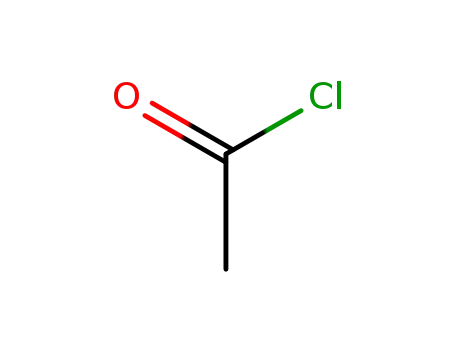
acetyl chloride

9-fluorenone
CAS:736928-22-6
CAS:884336-44-1