Your Location:Home >Products >Functional intermediates >123847-85-8

Product Details
Applications
N,N′-Di(1-naphthyl)-N,N′-diphenyl-(1,1′-biphenyl)-4,4′-diamine, also known as NPB or NPD, has been used intensively in OLEDs and other organic electronic devices such as polymer photovoltaics (OPV) and perovskite solar cells for its outstanding hole transport capability. NPB is considered as one of the best materials within its competition, and has become the most common-used material in OLEDs' application. This is due to its increased Tg up to 95 °C, which enhances device morphology and is beneficial for device longevity.
Description
N,N′-Di(1-naphthyl)-N,N′-diphenyl-(1,1′-biphenyl)-4,4′-diamine, also known as NPB or NPD, has been used intensively in OLEDs and other organic electronic devices such as polymer photovoltaics (OPV) and perovskite solar cells for its outstanding hole transport capability.
Chemical Properties
Light yellow to yellow powder
Uses
Hole transporting material for OLED devices
General Description
TGA/DSC Lot specific traces available upon request
Various triarylamines can be readily prepared in excellent yields by palladium-catalyzed cross-coupling reaction of aryl halides and diarylamines. The amination reaction takes place rapidly by using the catalyst combination of Pd(OAc)2 and a bulky and electron-rich ligands, P(t-Bu)3.
A series of N,N,N,N′-tetraaryl(Ar12Ar2 2)-1,1′-biphenyl-4,4′-diamine were synthesized by the Pd(0) catalyzed C-N bond formation methodology. The physical properties, including glass transition temperature (Tg), melting point (Mp) and oxidation potential were measured. For Ar1 = 2-naphthyl and Ar2 = 3-toly (4f) and Ar2 = 3-ethylphenyl(4h), the DSC charts indicate that they have Tg, but no observable Mp. These two compounds are amorphous in solid state. The X-ray powder diffraction pattern of 4f further confirmed that it is truly amorphous throughout the temperature range in which it is solid. Therefore, we have demonstrated that a low molecular weight organic solid can be amorphous. This is an important aspect in the preparation of morphologically stable amorphous thin film. Other compounds with Ar1 = Ph, 1-naphthyl while Ar2 = 3-toly or 3-ethylphenyl are normal compounds with both Tg and Mp when prepared in their glassy state by rapid cooling of their melt. The oxidation potentials for compounds with Ar1 = 1-naphthyl and 2-naphthyl and identical Ar2 are exactly the same. The molecular structural features must be important factors for an organic solid to be in the amorphous state.
The present invention relates to a novel compound, an organic electric element using the same, and an electronic device thereof. According to the present invention, a luminous efficiency, a color purity and a lifespan of the element can be improved and a driving voltage can be lowered. The organic electric element comprises: an anode; a cathode; and an organic material layer formed between the anode and the cathode.
Disclosed in the present application is an organic mixture. The organic mixture comprises two organic compounds H1 and H2. The organic compound H1 has electron transmission performance, and the organic compound H1 satisfies: Δ((LUMO+1)?LUMO)≥0.1 eV, and min((LUMO(H1)?HOMO(H2), LUMO(H2)?HOMO(H1))≤min(ET(H1), ET(H2)). The organic compound H1 and the organic compound H2 are easy to form exciplexes and have balanced electron transmission properties, the organic compound Hi has high stability of electron transmission, and accordingly the efficiency and the service life of related electronic components can be effectively improved, and a feasible solution for improving overall performance of the electronic components is provided.
The present invention provides a novel compound capable of improving the luminous efficiency, stability and life of an element, an organic electronic element using the same, and an electronic device comprising same.
A compound represented by Formula 1. An organic electric element includes a first electrode, a second electrode, and an organic material layer including the compound of Formula 1. The organic material layer include a light emitting layer, a hole transport layer including a compound represented by Formula 2, and an emission-auxiliary layer including the compound represented by Formula 1. When the organic electric element includes the compound in the organic material layer, luminous efficiency, color purity, and life span can be improved.
![{4-[(naphthalen-1-yl)(phenyl)amino]phenyl}boronic acid](/upload/2023/2/683f5170-686b-465b-9d2f-8c98c20a172b.png)
{4-[(naphthalen-1-yl)(phenyl)amino]phenyl}boronic acid

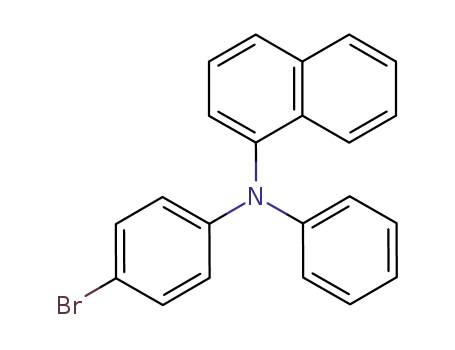
N-(4-bromophenyl)-N-phenyl-1-naphthylamine

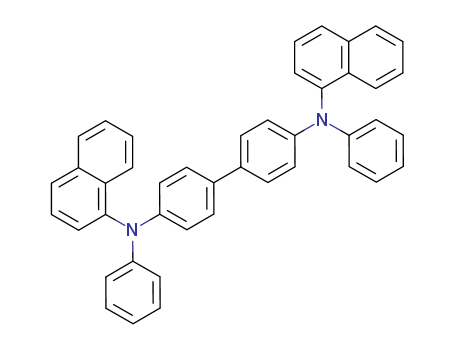
![4,4'-bis[N-(1-naphthyl)-N-phenylamino] biphenyl](/upload/2023/2/d910b85d-d97a-4278-8cd9-9e4e2a54785c.png)
4,4'-bis[N-(1-naphthyl)-N-phenylamino] biphenyl
| Conditions | Yield |
|---|---|
|
With
potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine;
In
water; toluene;
at 85 ℃;
for 24h;
Inert atmosphere;
|
95% |
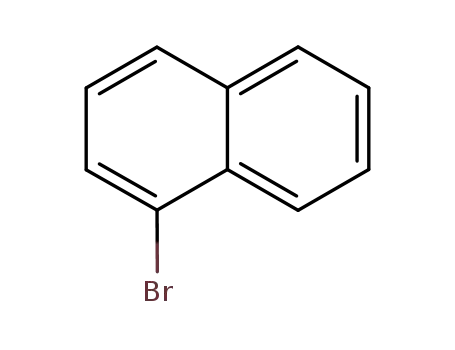
1-Bromonaphthalene

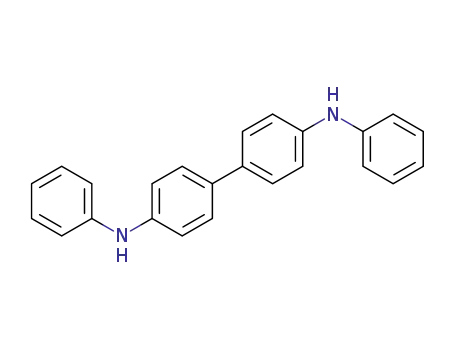
N,N'-diphenyl-p-phenylenediamine

![4,4'-bis[N-(1-naphthyl)-N-phenylamino] biphenyl](/upload/2023/2/d910b85d-d97a-4278-8cd9-9e4e2a54785c.png)
4,4'-bis[N-(1-naphthyl)-N-phenylamino] biphenyl
| Conditions | Yield |
|---|---|
|
With
sodium t-butanolate;
palladium diacetate; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride;
In
toluene;
at 20 - 130 ℃;
Inert atmosphere;
|
95% |
|
With
tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
for 12h;
Inert atmosphere;
Reflux;
|
70% |
|
With
1,1'-bis-(diphenylphosphino)ferrocene; sodium t-butanolate;
palladium diacetate;
In
toluene;
for 72h;
Heating;
|
54% |
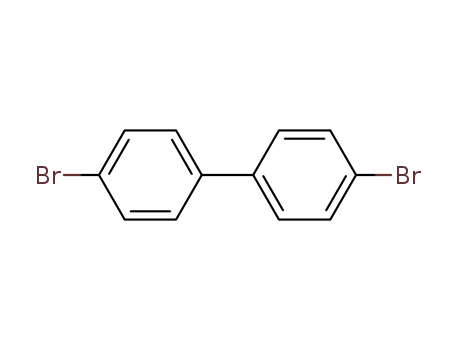
4-(4-bromophenyl)bromobenzene
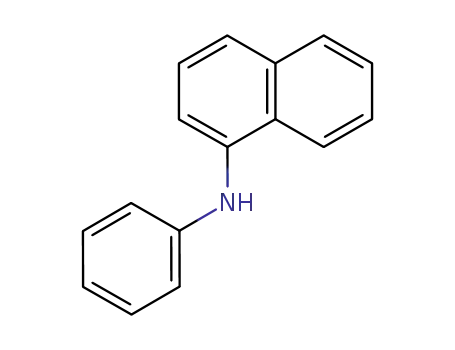
N-phenyl-1-naphthylamine

1-Bromonaphthalene

N,N'-diphenyl-p-phenylenediamine
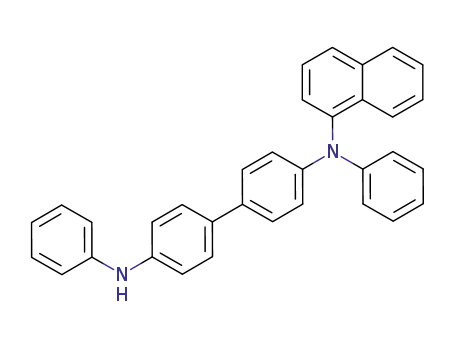
N-(1-Naphthyl)-N,N'-diphenylbenzidine
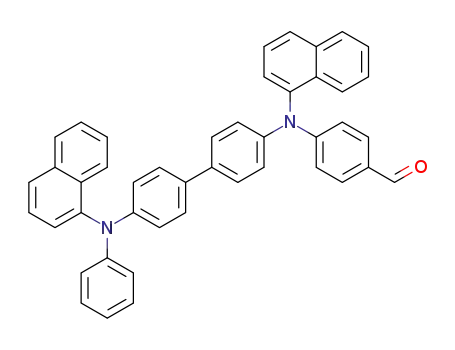
C45H32N2O
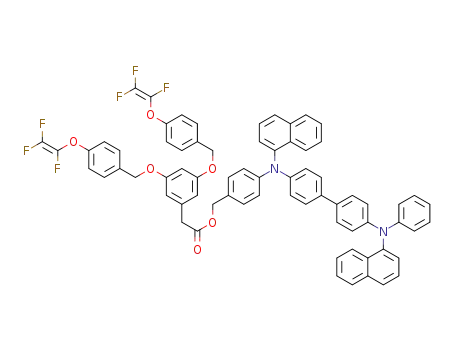
C71H50F6N2O6
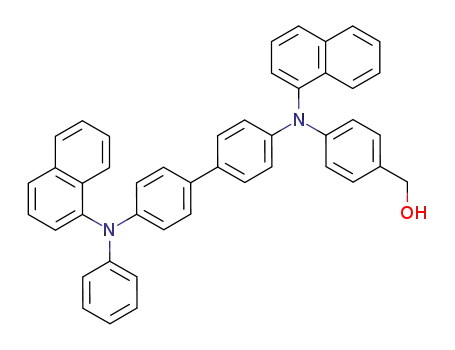
N,N'-di(1-naphthyl)-N-phenyl-N'-(4-(hydroxymethyl)phenyl)benzidine
CAS:6372-42-5
CAS:728039-63-2
CAS:152670-41-2