Your Location:Home >Products >OLED intermediates >Fluorenes >1016-05-3
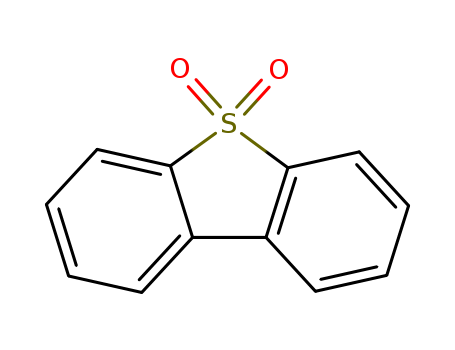

Product Details
Chemical Properties
white to yellow crystalline powder
Definition
ChEBI: A sulfone resulting from the oxidation of the sulfur atom of dibenzothiophene.
Synthesis Reference(s)
Tetrahedron Letters, 17, p. 785, 1976 DOI: 10.1016/S0040-4039(00)77951-2
InChI:InChI=1/C12H8O2S/c13-15(14)11-7-3-1-5-9(11)10-6-2-4-8-12(10)15/h1-8H
A SiO2-TiO2 mesoporous xerogel prepared in one-step by a non-hydrolytic route shows excellent performance in the mild oxidation of sulfides, sulfoxides and thiophenes with aqueous solutions of H 2O2. The Royal Society of Chemistry.
The present research has demonstrated that selective C?S bond cleavages of dibenzothiophene and its derivatives are feasible by thia-Baeyer–Villiger type oxidation, i. e. the oxygen insertion process within a sulfoxide-carbon linkage, in the presence of porphyrin iron (III) and by ultraviolet irradiation originating from sunlight, high pressure Hg-lamp or residentially germicidal ultraviolet lamp under very mild conditions. This reaction with tert-butylhydroperoxide at 30.0 °C leads to dibenzo[1,2]oxathiin-6-oxide (PBS) in 83.2 % isolated yield or its hydrated products, 2-(2-hydroxyphenyl)-benzenesulfinic derivatives (HPBS) in near 100 % yield based HPLC data. PBS and HPBS are a type of biological products detected on the C?S bond cleavage step through various oxidative biodesulfurization (OBDS) pathways, and are useful synthetic intermediates and fine chemicals. These observations may contribute on understanding delicately molecular aspect of OBDS in the photosynthesis system, expanding the C-S cleavage chemistry of S-heterocyclic compounds and approaching toward biomemic desulfurization with respect to converting sulfur contaminants to chemically beneficial blocks as needed and performing under the ambient conditions.
The removal of sulfur compounds from hydrocarbon-based distillate fuels is essential due to environmental concerns (i.e., acid rain and airborne particulate material production), and because a few parts per million of sulfur are enough to poison the catalysts used for the purification of the exhaust gases of diesel cars. The oxidation of dibenzothiophene (DBT) by H2O2 at 333 K was investigated using the catalysts (hydrotalcite (HT) and MgLa mixed oxide) and solvents (methanol, acetonitrile, benzonitrile, acrylonitrile, and 3-methoxypropionitrile). High activity was found only after calcinations followed by rehydration of HT. Acetonitrile was the best solvent, while much lower reaction rates were observed in methanol. The decomposition of H2O2 into oxygen was observed for HT, and was the major reaction at > 353 K. This reaction did not occur in the absence of nitrile, and was faster over MgLa mixed oxide of higher basic strength. The activity increased with increasing Mg/Al ratio due to a lower rate of H2O2 decomposition attributed to a lower basicity of the solid.
Aiming at the deep desulfurization of the diesel oil, a comparison of the catalytic effects of several Keggin type POMs, including H3PWxMo12-xO40 (x = 1, 3, 6), Cs2.5H0.5PW12O40, and H3PW12O40, was made, using the solution of DBT in normal octane as simulated diesel oil, H2O2 as oxidant, and acetonitrile as extractant. H3PW6Mo6O40 was found to be the best catalyst, with a desulfurization efficiency of 99.79% or higher. Hence, it is promising for the deep desulfurization of actual ODS process. The role of the main factors affecting the process including temperature, O/S molar ratio, initial sulfur concentration, and catalyst dosage, was investigated, whereby the favourable operating conditions were recommended as T = 60 °C, O/S = 15, and a catalyst dosage of 6.93 g (H3PW6Mo6O40)/L (simulated diesel). With the aid of GC-MS analysis, sulfone species was confirmed to be the only product after reaction for 150 min. Furthermore, macro-kinetics of the process catalyzed by H3PW6Mo6O40 was studied, from which the reaction orders were found to be 1.02 to DBT and 0.38 to H2O2, and the activation energy of the reaction was found to be 43.3 kJ/mol. Moreover, the catalyst recovered demonstrated almost the same activity as the fresh.
A simple thermo-responsive one-phase microemulsion (μem) is designed to enable the dark singlet oxidation of organic substrates while allowing a straightforward separation of the catalytic surfactant and products in two distinct phases by cooling down the reaction medium. This latter is prepared by combining a small amount (1%) of the catalytic surfactant bis(dimethyldioctylammonium) molybdate, [DiC8]2[MoO4], with the nonionic amphiphile tetraethylene glycol monooctyl ether, C8E4. Tensiometry and dynamic light scattering are used to rationalize the synergy between the two surfactants which strongly interact. The oxidation takes place in the effective one-phase Winsor IV system which separates into two phases (μem + oil, i.e. Winsor I) just by temperature change thanks to the presence of the thermosensitive C8E4. The thermal-controlled nanostructured reaction medium is applied to the ene reaction, [4+2] cycloaddition and sulfide oxidation.
With the issues of increasingly stringent legislations on sulfur level in transportation fuels, clean fuels research including desulfurization has become a more important subject of environmental catalysis studies. Oxidative desulfurization (ODS) combined with extraction is one of the most promising desulfurization processes. With the loading of Cs2.5H0.5PW12O40, a new multi-walled carbon nanotube (MWNT) supported catalyst (Cs2.5H0.5PW12O40/MWNT) has been developed in this study. Through experimental evaluations, Cs2.5H0.5PW12O40/MWNT was found to be very effective for the oxidative removal of DBT, with a desulfurization efficiency of up to 100%. Factors affecting the process, including reaction temperature, O/S molar ratio, initial sulfur content, catalyst dosage, and pre-immersion time of the catalyst in H2O2 solution, were evaluated, and the favourable operating conditions were determined. Sulfone species was confirmed by GC-MS analysis to be the only product from DBT oxidation by H2O2 in the presence of Cs2.5H0.5PW12O40/MWNT after 160 min. Moreover, the new catalyst developed is recoverable. The recovered Cs2.5H0.5PW12O40/MWNT demonstrates quite close catalytic activity to that of the fresh. As a whole, Cs2.5H0.5PW12O40/MWNT has a high potential for using as an effective catalyst for deep desulfurization of diesel.
Benzothiophene (BT), dibenzothiophene (DBT) and 4,6-dimethlydibenzothiophene (4,6-DMDBT) were extracted from an oil phase to ionic liquid phase, and then oxidized to the corresponding sulfone by the cheap catalyst, N-hydroxyphthalimide (NHPI), using molecular oxygen as the oxidant in one-pot. The system can be recycled 5 times without a significant decrease in desulfurization.
A variety of symmetrical and unsymmetrical sulfides have been selectively and expeditiously oxidized to either sulfoxides or sulfones in good yields using wet silica-supported sodium periodate under microwave thermolysis conditions.
An effcient and reusable molybdenum-based catalyst has been prepared by tethering dioxomolybdenumacetylacetonate complex, MoO2(acac)2, via postsynthesis modifcation of zeolite beta. The catalyst has been characterized by Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy-energy dispersive X-ray analysis (SEM-EDX) and inductively coupled plasma (ICP). The catalyst exhibited very high activity for the selective oxidation of sulfdes to sulfones at room temperature. The catalyst can be recycled and reused four times without signifcant loss of activity.
-
Dibenzothiophenes were oxidized effectively with H2O 2 in the presence of 12-tungstophosphoric acid in the tetradecane/ AcOH biphasic system to give their corresponding sulfones as the major products. The oxidation proceeded in the AcOH phase and most of the sulfones distributed there, resulting in the successive removal of the sulfur compounds from the tetradecane phase. This biphasic oxidation system can effectively reduce the sulfur content in light oil.
Catalytic oxidation of dibenzothiophene (DBT) in decalin was performed using an oil-soluble oxidant, cumene hydroperoxide (CHP), over molybdenum oxide (MoO3) supported on silica. The effects of MoO3 loading, reaction time and the molar ratio of CHP/DBT were investigated. At a MoO3 loading of 15 wt%, the conversion of DBT reached 82% at 70 °C, WHSV 30 h-1, and O/S molar ratio 3. Alkaline earth metals, such as Ca, Ba, Sr and Mg were introduced on the surface of silica, prior to the impregnation of MoO3. The results showed that the activity in the oxidation of DBT with CHP decreased in the order: MoO3/Ca-SiO2 > MoO3/Ba-SiO2 > MoO3/SiO2 > MoO3/Sr-SiO2 > MoO3/Mg-SiO2. The MoO3/Ca-SiO2 catalysts were characterized by XRD. The DBT conversions on MoO3/Ca-SiO2 catalysts with various Ca/Mo ratios were studied. When the Ca/Mo ratio was 0.05, the DBT conversion was the highest (95%) at 60 °C, WHSV 30 h-1, and O/S molar ratio 3.0.
The polyoxometalate (POM) catalyst, [γ-SiW10O 34(H2O)2]4-, was introduced into the pores of both as-synthesized (I) and amine functionalized MCM-41 (II). The resultant catalysts were characterized with powder X-ray diffraction, nitrogen sorption, and diffuse-reflectance UV-vis spectroscopy. Both catalysts were tested for reusability through repeated catalytic conversions of methyl phenyl sulfide to methyl phenyl sulfone with hydrogen peroxide. While the physisorbed catalyst (I) exhibits steadily decreasing turnover frequency (TOF), the POM catalyst supported on MCM-41 functionalized with a protonated amine (II) exhibits markedly improved reusability. This chemisorbed catalyst effectively showed no change in TOF between the second (21) and the sixth reactions (22). Additionally, sulfoxidations with catalyst II were investigated with a small set of substrates focusing on compounds including dibenzothiophene, which serves as a model refractory sulfide.
A facile approach to the preparation of a novel magnetically separable H5PMo10V2O40/Fe3O4/g-C3N4 (PMoV/Fe3O4/g-C3N4) nanocomposite by chemical impregnation is demonstrated. The prepared nanocomposite was characterized and its acidity was measured by potentiometric titration. PMoV/Fe3O4/g-C3N4 showed high catalytic activity in the selective oxidative desulfurization of sulfides to their corresponding sulfoxides or sulfones. The catalytic oxidation of a dibenzothiophene (DBT)-containing model oil and that of real oil were also studied under optimized conditions. In addition, the effects of various nitrogen compounds, as well as the use of one- and two-ring aromatic hydrocarbons as co-solvents, on the catalytic removal of sulfur from DBT were investigated. The catalyst was easily separated and could be recovered from the reaction mixture by using an external magnetic field. Additionally, the remaining reactants could be separated from the products by simple decantation if an appropriate solvent was chosen for the extraction. The advantages of this nanocatalyst are its high catalytic activity and reusability; it can be used at least four times without considerable loss of activity.
The Bronsted acidic ionic liquids 1-butyl-3-methylimidazolium hydrogen sulfate ([BMIM][HSO4]) and N-butylpyridinium hydrogen sulfate ([C4Py][HSO4]) were used as extractant and catalyst for desulfurization of diesel. The results show that [BMIM][HSO 4] is better as extractant and catalyst than [C4Py] [HSO4] during the desulfurization process. The sulfur removal of dibenzothiophene (DBT) in n-octane was 99.6% in 90 min under the conditions of Vmodel oil/VIL = 2:1 and H2O2/DBT molar ratio at 5 (O/S = 5), at room temperature. The sulfur removal of four sulfur compounds by extraction and catalytic oxidation process followed the order of DBT > benzothiophene (BT) > thiophene (TS) > 4,6-dimethyldibenzothiophene (4,6-DMDBT). Moreover, the [BMIM][HSO4] can be recycled for at least 6 times with a little decrease in the desulfurization activity. The sulfur removal of diesel fuel containing sulfur content of 97 ppm is 85.5%, which was much better than desulfurization performance by simple extraction with IL (11.0%). In this extraction and oxidative desulfurization process, DBT was oxidized to corresponding sulfone by H2O2 with Bronsted acidic IL [BMIM][HSO 4] which served as not only extractant but also catalyst. The Royal Society of Chemistry 2010.
Organosulphur compounds can be easily and selectively oxidized to sulfones using a small excess of Oxone (1.6 eq.) under solventless mechanical milling conditions. This green procedure has been efficiently applied to a series of model compounds and to the desulphurization of medium/high sulphur content paraffins (up to 3000 mg kg-1).
Aromatic and aliphatic sulfides are oxidized to sulfoxides or sulfones in high yield with 30% hydrogen peroxide under organic solvent- and halogen-free conditions. Dialkyl and alkyl aryl sulfides are cleanly oxidized to sulfoxides using aqueous hydrogen peroxide without catalysts. The best catalyst for the sulfone synthesis consists of sodium tungstate, phenylphosphonic acid, and methyltrioctylammonium hydrogensulfate. Co-existing primary or secondary alcohol or olefinic moieties are unaffected under such conditions.
Changes in the expression of α7 nicotinic acetylcholine receptors (α7 nAChRs) in the human brain are widely assumed to be associated with neurological and neurooncological processes. Investigation of these receptors in vivo depends on the availability of imaging agents such as radioactively labelled ligands applicable in positron emission tomography (PET). We report on a series of new ligands for α7 nAChRs designed by the combination of dibenzothiophene dioxide as a novel hydrogen bond acceptor functionality with diazabicyclononane as an established cationic center. To assess the structure-activity relationship (SAR) of this new basic structure, we further modified the cationic center systematically by introduction of three different piperazine-based scaffolds. Based on in vitro binding affinity and selectivity, assessed by radioligand displacement studies at different rat and human nAChR subtypes and at the structurally related human 5-HT3 receptor, we selected the compound 7-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-2-fluorodibenzo-[b,d]thiophene 5,5-dioxide (10a) for radiolabeling and further evaluation in vivo. Radiosynthesis of [18F]10a was optimized and transferred to an automated module. Dynamic PET imaging studies with [18F]10a in piglets and a monkey demonstrated high uptake of radioactivity in the brain, followed by washout and target-region specific accumulation under baseline conditions. Kinetic analysis of [18F]10a in pig was performed using a two-tissue compartment model with arterial-derived input function. Our initial evaluation revealed that the dibenzothiophene-based PET radioligand [18F]10a ([18F]DBT-10) has high potential to provide clinically relevant information about the expression and availability of α7 nAChR in the brain.
A moisture-tolerant cyclopentadienyl-silsesquioxane titanium complex efficiently catalyses the selective oxidation of various types of sulfides to either sulfoxides (TOFs up to 32 530 h-1) or sulfones with H2O2 (30% in water) under mild conditions.
An ammonium salt of molybdenum heteropoly acid was loaded on silica support (HPA-SiO2), used as catalyst in oxidative desulfurization for removing benzothiophene (BT), dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) from model oil. The structural properties of prepared catalyst were studied by using various analytical techniques; Fourier transforms infrared spectroscopy, X-ray diffraction, scanning electron microscopy and thermogravimetric analysis. The results showed that the ammonium salt of molybdenum heteropoly acid highly dispersed on SiO2. The final catalyst exhibits good catalytic activity of oxidative desulfurization. The 94.6 % of thiophene compounds of the model oil were removed under optimal conditions; temperature at 80 °C, reaction time 100 min. The oxidation is decreased in order of DBT > 4,6-DMDBT > BT.
-
-
Three cobalt(II)-salen complexes (CoL1, CoL2 and CoL3) were synthesized via the reaction of the three tetradentate ligands as N,N′-ethylenebis(salicylimine) L1, N,N′-ethylenebis(3-tert-butylsalicylimine) L2 and N,N′-ethylenebis(3,5-di-tert-butylsalicylimine) L3, with a stoichiometric amount of cobalt(II) acetate tetrahydrate, respectively. All the three complexes were studied as oxidative desulfurization catalyst on dibenzothiophene taken in model fuel oil n-dodecane. The acetonitrile used as an extracting solvent and H2O2 as an oxidant. Comparatively CoL3 proved to be the best catalyst which showed the 76% DBT removal at the optimized conditions. The nth-order kinetic model is the best way to represent oxidation kinetics of complexes. Graphic Abstract: [Figure not available: see fulltext.]This cobalt(II) Schiffs base complexes were studied as catalyst for oxidative desulfurization of dibenzothiphene (DBT) taken as model sulphur compounds in fuel model oil (n-dodecane) using H2O2 as oxidant and acetonitrile as extracting solvent for DBT sulfone in a batch experiment.
A new reaction-controlled phase-transfer catalyst based on silicotungstate of [(C18H37)2(CH3) 2N]3[SiO4H(WO5)3] for oxidation of hydrocarbons is developed. The catalyst is a new heteropoly compound with silicon as heteroatom, which is different to the previously reported reaction-controlled phase transfer catalysts that were composed of quaternary ammonium heteropolyoxotungstates of [π-C5H 5N(CH2)15CH3]3[PW 4O16] and [π-C5H5N(CH 2)15CH3]3[PW4O 32] with phosphorus as heteroatom. The oxidation of various alkenes (such as linear terminal olefins, internal olefins, cyclic olefins and unactivated alkenes) to epoxides, sulfides to sulfoxides and sulfones, alcohols to carbonyl compounds, are successfully catalyzed by this recyclable and environmentally benign catalyst using H2O2 as oxidant and ethyl acetate as solvent. This catalyst is not only capable of catalyzing homogeneous oxidation of organic substrates with unique reaction-controlled phase-transfer character, but also avoids the use of toxic solvents. The catalyst could be easily recovered and reused after reaction, and the epoxidation of cyclohexene was performed twenty times without obvious loss in activity. The fresh catalyst and the used one were characterized by ICP, IR, UV-vis, 29Si MAS NMR and 183W NMR in detail. the Partner Organisations 2014.
We have designed and synthesized two novel yellow phosphorescent iridium complexes using dibenzothiophene-S,S-dioxide-based cyclometalated ligand for the first time, which are capable of producing highly efficient yellow and white polymer light-emitting devices (PLEDs). The resulted iridium complexes display good thermal stability and high photoluminescence quantum yields. Both yellow and white PLEDs are fabricated with an identical single-emission-layer configuration of ITO/PEDOT:PSS/emission layer (EML)/CsF/Al. For the yellow phosphorescent PLEDs based on (p-CzFSOPy)2IrPic, the best device performances with a peak luminous efficiency (LE) of 13.3 cd/A and a peak external quantum efficiency (EQE) of 5.3% are achieved. More importantly, the two-element WPLEDs containing iridium (III)bis (2-(4,6-difluorophenyl)-pyridinato-N,C2′) picolinate (FIrpic) as blue and (p-CzFSOPy)2IrPic as yellow phosphors doped into a PVK:OXD-7 matrix at an appropriate ratio exhibited a maximum LE of 19.2 cd/A, a maximum EQE of 9.6%, an extremely high luminance of 18717 cd/m2 and Commission Internationale de L'Eclairage (CIE) coordinate of (0.317, 0.448). Moreover, at a luminance for practical application of 1000 cd/m2, the LE still remains as high as 19.0 cd/A, with a very slight decrease.
A practical and environmentally benign electrochemical oxidation of thioethers and thiols in a commercially-available continuous-flow microreactor is presented. Water is used as the source of oxygen to enable the oxidation process. The oxidation reaction utilizes the same reagents in all scenarios and the selectivity is solely governed by the applied potential. The procedure exhibits a broad scope and good functional group compatibility providing access to various sulfoxides (15 examples), sulfones (15 examples) and disulfides (6 examples). The use of continuous flow allows the optimal reaction parameters (e.g. residence time, applied voltage) to be rapidly assessed, to avoid mass- and heat-transfer limitations and to scale the electrochemistry.
-
The tetrabutylammonium (TBA) salt of a Keggin-type polyoxometalate (POM), with the chemical formula TBA4H2[BW11Mn(H2O)O39]·H2O, TBABW11Mn, was evaluated as a catalyst in the oxidation by hydrogen peroxide of several organosulfur compounds, namely benzothiophene (BT), 2-methylbenzothiophene (2-MBT), 3-methylbenzothiophene (3-MBT), dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT) and 4,6-diethyldibenzothiophene (4,6-DEDBT), in acetonitrile at room temperature. All compounds were oxidized to their corresponding sulfones with high conversion and selectivity. Following the excellent results achieved, the BW11Mn/H2O2 in CH3CN system was tested in the oxidation of model fuels (MFs) consisting of a mixture of BTs and DBTs in hexane (MF1 containing mainly BTs and MF2 containing predominantly DBTs). In both cases, the organosulfur compounds from the model fuels were fully converted into their corresponding sulfones. Envisaging the development of a promising desulfurization procedure, the extraction of sulfur compounds from MF2 was attempted after the catalytic oxidation process. More than 98 mol% was removed using an ethanol/H2O mixture.
-
Three hydrophobic Keggin-type heteropolyacid catalysts, [C 3H3N2(CH3)(C2H 4)]5PMo10V2O40 ([C 2mim]PMoV), [C3H3N2(CH 3)(C4H8)]5PMo10V 2O40 ([C4mim]PMoV) and [C3H 3N2(CH3)(C6H12)] 5PMo10V2O40 ([C6mim]PMoV) , were synthesized by reacting molybdovanadophosphoric acid with imidazolium bromides, and characterized by spectroscopic methods. Their use as catalysts in the extractive catalytic oxidative desulfurization process using hydrogen peroxide as the oxidant and acetonitrile as phase transfer agent was studied. The catalytic properties decreased in the order: [C6mim]PMoV > [C4mim]PMoV > [C2mim]PMoV. The main factors influencing the rate of removal of dibenzothiophene (DBT) were investigated, including reaction temperature, the amounts of catalyst, H2O2 and acetonitrile. Nearly 100 % sulfur removal rate was achieved under optimal conditions. The catalyst could be recycled six times with only a slight decrease in activity. A reaction mechanism for DBT oxidation is proposed, in which the Keggin anions first obtain active oxygen from H2O2, then the DBT is oxidized to dibenzothiophene sulfones.
Mild and safe oxidation of dialkyl-, diaryl- and alkyl-aryl sulfides (1a-m) to the corresponding sulfones (2a-m)by using urea-hydrogen peroxide/formic acid system is reported. Wiley-VCH Verlag GmbH, 1999.
The acetylacetonate complexes of oxovanadium(IV): VO(X-acac)2 (X = Cl, CH3 and CH3CH2) were structurally characterized in several alkylimidazolium ionic liquids by visible absorption and EPR spectroscopies. VO(Me-acac)2 and VO(Et-acac)2 showed solvatochromism in the selected ionic liquids (a λ1 shift of ca. 120 nm was observed) and most complexes showed two species in the anisotropic EPR spectra in several ionic liquids. It was shown that the ionic liquids anions have coordinating ability and the basicity order found was: NTf2- - 4 -, in agreement with previous findings. An extraction and catalytic oxidation desulfurization (ECODS) system composed of VO(X-acac)2, H2O2 and ionic liquids was tested in the removal of benzothiophene (BT), dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) from a model oil (n-octane) at ambient temperature. The influence of the catalyst, ionic liquid, substrate and the molar ratio of the oxidant (H2O2) and the S-containing molecule were examined. The reaction rates of the oxidative desulfurization reaction were found to increase with the molar ratio of H2O2 and the S-containing molecule, as expected, however a ratio of 4 was enough to achieve >95% conversion to sulfone. The ionic liquid that showed the best performance with all substrates was bmimNTf2. In bmimOTf and bmimBF4 the activity of all catalysts in the ECODS system was low to moderate, which is probably due to the higher coordinating ability of these anions.
Mesoporous silica-titania materials of tunable composition and texture, which present a high catalytic activity in the mild oxidation of sulfur compounds, have been obtained by combining the spray-drying process with the colloidal self-assembly of α-chitin nanorods (biopolymer acting as a template) and organometallic oligomers.
A series of imidazolium perrhenate ionic liquids (IPILs) have been synthesized and used as efficient catalysts for the oxidation of sulfides to sulfones with aqueous hydrogen peroxide under mild conditions. Various sulfones are obtained in good to excellent yields. IPILs are stable and can be reused at least ten times without detectable activity loss. Furthermore, this system provides a reasonably environmentally benign chemical process in terms of potential industrial application.
An innovative approach for desulfurisation of fuels is proposed. It relies on the formation of recognition sites, complementary to oxidized sulfur-containing compounds, on cross-linked chitosan microspheres and electrospun chitosan nanofibers using the molecularly imprinted polymer technique. Benzothiophene sulfone (BTO2), dibenzothiophene sulfone (DBTO2) and 4,6-dimethyldibenzothiophene sulfone (4,6-DMDBTO 2) were used as templates for the preparation of molecularly imprinted polymers (MIPs). The possible molecular interactions between imprinted chitosan adsorbent and oxidized sulfur-containing compounds were investigated by molecular modeling using density functional theory (DFT) and results indicated that interactions took place via hydrogen bonding. The molecularly imprinted polymer adsorbents (cross-linked microspheres and electrospun nanofibers) gave better selectivity for the target sulfonated compounds and the adsorption isothermal studies followed the Freundlich model. Maximum adsorption capacities of 8.5 ± 0.6 mg/g, 7.0 ± 0.5 mg/g and 6.6 ± 0.7 mg/g were observed for model BTO2, DBTO2 and 4,6-DMDBTO2 respectively at 1 mL/h when imprinted nanofibers were employed, and the imprinted microspheres gave maximum adsorption capacity of 4.9 ± 0.5 mg/g, 4.2 ± 0.7 mg/g and 3.9 ± 0.6 mg/g for BTO 2, DBTO2 and 4,6-DMDBTO2 respectively. Application of the nanofibers to oxidized hydro-treated fuel under continuous flow adsorption system at 1 mL/h indicated that 84% of sulfur was adsorbed, with adsorption capacity of 2.2 ± 0.2 mg/g.
The binuclear Mn(IV)-Mn(IV) manganese complex 1 catalyzes the periodic acid oxidation of sulfides to sulfones under mild conditions. The reaction was found to be highly selective giving almost quantitative yields of the sulfones even in the presence other easily oxidized groups. Only amines were found to hinder the reaction.
A series of task-specific acidic ionic liquids (TSILs), immiscible with oil, halogen-free and containing -COOH group in the cations, were used for oxidative desulfurization as both the catalyst and extractant. The removal of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) from model diesel at 298 K could reach 96.7% and 95.1%, respectively. The TSIL could be recycled 5 times without any apparent loss of the catalytic activity. Meanwhile, the structures, acidities and interactions between the cation and the anion of TSILs have been investigated by density functional theory (DFT) method, and found that catalytic properties of TSILs are close related to the structures, acidities and extraction capabilities. Furthermore, an oxidative desulfurization mechanism has been proposed.
Selective, mild and safe oxidation of dialkyl-, diaryl- and alkyl-aryl sulfides to the corresponding sulfones was achieved by using of urea- H2O2/trifluoroacetic anhydride system.
It was first shown that crown ethers catalyze the oxidation of organic sulfur compounds (methyl phenyl sulfide to sulfoxide and sulfone, benzothiophene and dibenzothiophene to sulfones) with hydrogen peroxide. Nauka/Interperiodica 2007.
Vanadium supported-CMK-3 catalysts with vanadium loading of 1–7?wt.% were studied in the oxidative desulfurization (ODS) of dibenzothiophene as a model sulfur compound. The activity was compared with titanium supported-CMK-3. Structural and textural characterization of the catalysts was performed by means of N2 adsorption, XRD, UV–vis–DRS, Raman spectroscopy, XPS, TEM and TPR. The dispersion and the nature of the vanadium species depend on the V loading, so does the catalyst activity. Vanadium supported-CMK-3 with 7?wt.% of vanadium loading was the most active catalyst for ODS of DBT using hydrogen peroxide (H2O2) as oxidant and acetonitrile as solvent. 100% of DBT elimination was attained at short time in mild conditions. Carbon ordered mesoporous CMK-3 with high surface area and high pore volume promotes a very good anchorage of metallic oxides in the carbons framework reaching high active sites distribution and more stable nanoclusters. The reusability of the catalyst indicates that V-CMK-3 is a potential catalyst for the ODS of dibenzothiophene.
The structure of IRMOF-3 was modified with pyridine-2-aldehyde and then the Schiff base moieties were used to anchor ruthenium complex to this metal-organic framework. The prepared catalyst was characterized by X-ray powder diffraction (XRD), diffuse reflectance spectroscopy (DRS), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and FTIR methods. The results show that the prepared catalyst acquires a high potential for selective oxidation of aromatic and heteroaromatic compounds under mild conditions and easy workups.
Nanometre-sized metal oxides are promising species for the development of visible-light-responsive photocatalysts for the selective transformation of organic functional groups. In this article, we report that decavanadate ([V10O28]6-, V10) behaved as an efficient visible-light-responsive photocatalyst in the product-selective oxygenation of sulfides achieved using O2 (1 atm) as the green oxidant. In particular, we revealed that visible-light-responsive photocatalysis of V10 showed remarkable activity for the oxygenation of structurally diverse sulfides to form the corresponding sulfones using O2 in methyl ethyl ketone (MEK). Furthermore, by simply adding water to the reaction mixture, the product selectivity of sulfide oxygenation can be significantly switched toward the production of sulfoxides, without concomitant loss of photocatalytic activity. Based on experimental evidence, we inferred the following mechanistic steps for this photocatalytic system: the aerobic oxygenation of sulfides to form the corresponding sulfoxides initiated by a visible-light-induced photoredox reaction of V10. As for the formation of sulfones, MEK-derived peroxide species as the co-catalysts are probably involved in the oxygenation of sulfoxides to sulfones. The selectivity switch of the V10-photocatalysed reaction brought about by water addition is most likely achieved by suppressing the formation of MEK-derived peroxide species. This journal is
The oxidation of organo-sulfides to sulfones has been accomplished using an easily accessible bifunctional ionic liquid, [pmim]IO4 in the absence of any other oxidants, metal and organic solvent at ambient temperature. A variety of sulfides including dialkyl, aryl-alkyl, diaryl, and aryl-hetero aryl have been oxidized to the corresponding functionalized sulfones in high yields by this procedure.
A new vanadoperiodate [HIV9O28]3- has been synthesized by a simple, one-pot stoichiometric reaction of HIO4·2H2O with NaVO3 in aqueous solution and then isolated as [C8H17/
Straight-run diesel fuel containing 1.6% of sulfur was enzymatically oxidized with chloroperoxidase from Caldariomyces rumago. Most organosulfides and thiophenes were transformed to form sulfoxides and sulfones. The oxidized organosulfur compounds can be effectively removed by distillation. The resulting fraction after distillation contained only 0.27% sulfur, while the untreated straight-run diesel fuel after the same distillation process still showed 1.27% sulfur. To know the chemical nature of the products, nine organosulfur compounds and 12 polycyclic aromatic compounds (PACs) were transformed by chloroperoxidase in the presence of chloride and hydrogen peroxide. Organosulfur compounds were only oxidized to form sulfoxides and sulfones, and no chlorinated derivatives were detected, except for bithiophene. In contrast, PACs were exclusively chlorinated, and no oxidized derivatives could be found. No enzymatic activity was detected on PACs with an ionization potential higher than 8.52 eV, while in the lower region it was found that the higher the ionization potential of the PAC the lower the specific activity. On the other hand, the substrate ionization potential did not seem to influence chloroperoxidase activity in the oxidation of organosulfur compounds. All organosulfur compounds tested were oxidized by chloroperoxidase. From double-substrate experiments, it appears that organosulfur compounds are oxidized by both compound I and compound X enzyme intermediates, while PACs react only with the halogenating intermediate, compound X. Straight-run diesel fuel containing 1.6% of sulfur was enzymatically oxidized with chloroperoxidase from Caldariomyces fumago. Most organosulfides and thiophenes were transformed to form sulfoxides and sulfones. The oxidized organosulfur compounds can be effectively removed by distillation. The resulting fraction after distillation contained only 0.27% sulfur, while the untreated straight-run diesel fuel after the same distillation process still showed 1.27% sulfur. To know the chemical nature of the products, nine organosulfur compounds and 12 polycyclic aromatic compounds (PACs) were transformed by chloroperoxidase in the presence of chloride and hydrogen peroxide. Organosulfur compounds were only oxidized to form sulfoxides and sulfones, and no chlorinated derivatives were detected, except for bithiophene. In contrast, PACs were exclusively chlorinated, and no oxidized derivatives could be found. No enzymatic activity was detected on PACs with an ionization potential higher than 8.52 eV, while in the lower region it was found that the higher the ionization potential of the PAC the lower the specific activity. On the other hand, the substrate ionization potential did not seem to influence chloroperoxidase activity in the oxidation of organosulfur compounds. All organosulfur compounds tested were oxidized by chloroperoxidase. From double-substrate experiments, it appears that organosulfur compounds are oxidized by both compound I and compound X enzyme intermediates, while PACs react only with the halogenating intermediate, compound X.
In this work, ultrasound-assisted oxidative desulfurization (UAOD) of liquid fuels performed with a novel heterogeneous highly dispersed Keggin-type phosphotungstic acid (H3PW12O40, PTA) catalyst that encapsulated into an amino-functionalized MOF (TMU-17-NH2). The prepared composite exhibits high catalytic activity and reusability in oxidative desulfurization of model fuel. Ultrasound-assisted oxidative desulfurization (UAOD) is a new way to performed oxidation reaction of sulfur-contain compounds rapidly, economically, environment-friendly and safely, under mild conditions. Ultrasound waves can be apply as an efficient tool to decrease the reaction time and improves oxidative desulfurization system performance. PTA@TMU-17-NH2 could be completely performed desulfurization of the model oil by 20?mg of catalyst, O/S molar ratio of 1:1 in presence of MeCN as extraction solvent. The obtained results indicated that the conversions of DBT to DBTO2 achieve 98% after 15?min in ambient temperature. In this work, we prepared TMU-17-NH2 and PTA/TMU-17-NH2 composite by ultrasound irradiation for first time and employed in UAOD process. Prepared catalyst exhibit an excellent reusability without PTA leaching and loss of activity.
In this work, the new catalyst (assigned as TBAPW11Zn?TiO2?PAni) was successfully designed and synthesized on the basis of quaternary ammonium salt of zinc monosubstituted phosphotungstate [(n-C4H9)4N][PW11ZnO39] (TBAPW11Zn), titanium dioxide (TiO2), and polyaniline (PAni). This study reports the catalytic oxidation-extraction desulfurization (ECODS) of sulfur-containing molecules from real and the simulated (Th, BT, and DBT) gasoline using new organic–inorganic hybrid catalyst (TBAPW11Zn?TiO2?PAni). The ECODS results were shown that the concentration of sulfur compounds (SCs) of real gasoline was lowered from 0.4992 to 0.0122 wt.% with 97% efficiency at 35 °C after 1 h. Furthermore, the synthesized heterogeneous nanocatalyst showed high stability and reusability after five times without significant loss of activity. The high performance of TBAPW11Zn?TiO2?PAni/H2O2/CH3CO2H system can be a promising route with a superb potential in the generation of ultra-low-sulfur gasoline. Also the Mann–Whitney U-test results show that there is not a significant difference between the mean of sulfur percentage for DBT & BT, BT & Th and DBT & Th in the presence of the catalyst. Based on the Kruskal–Wallis test results, we can conclude that the temperature, time and amount of catalyst have a significant effect on ECODS efficiency of TBAPW11Zn?TiO2?PAni nanocomposite.
This paper describes the oxidation of several model S-containing molecules with hydrogen peroxide in L-L phase system using a heterogeneous catalyst under atmospheric pressure in the 333-353 K temperature range. Molybdenum and tungsten compounds are prepared by anion exchange with alkylammonium derivatives covalently anchored to silica gel. These solids are robust heterogeneous catalysts able to activate selectively hydrogen peroxide to remove sulfur compounds via oxidation (ODS). The influence of several reaction variables (the reaction temperature, the nature of the substrate, the solvent, the molar ratio of the oxidant (H2O2), the S-containing molecule, the catalysts nature and the reuse of the catalysts) on the performance was examined. The potential of this methodology is illustrated by the complete S removal from a 0.2 wt% dibenzothiophene mixture at 353 K in less than 1 h of reaction. Molybdenum catalysts exposed to hydrogen peroxide form peroxomolybdates moieties which are more active than acid precursors. The activated Mo catalysts are very active in ODS reaction and can be reused four times without lose of activity.
The development of poly[allylSB-co-EGDMA] beads containing a tetradentate ligand was achieved via suspension polymerization. The catalyst poly[allylSB-co-EGDMA]-VO was synthesized by reacting VIVOSO 4 with poly[allylSB-co-EGDMA]. XPS
The wet impregnation method was applied to prepare vanadium species on the chromium(III) terephthalate metal-organic framework (MIL-101) and on the aluminum fumarate metal-organic framework (A520), two highly efficient catalysts, as well as vanadium species on the zinc terephthalate metal-organic framework (MOF-5), using vanadium(V) oxytributoxide as a precursor. The prepared catalysts were characterized by powder X-ray diffraction (PXRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDX), N2 sorption measurement (BET), thermal analysis (TGA-DTA), and X-ray photoelectron spectroscopy (XPS). The prepared materials were utilized as catalysts for the oxidation of the refractory aromatic sulfur compound, dibenzothiophene (DBT), in a model diesel fuel. The catalytic oxidation of DBT to DBT-sulfone was performed in the presence of tert-butyl hydroperoxide (TBHP) under mild operating conditions. The heterogeneous catalysts, OV(OtBu)3?x(OH)x@MIL-101(Cr), OV(OtBu)3?x(OH)x@A520, and OV(OtBu)3?x(OH)x@MOF-5 demonstrated activities of 98, 98, and 10 %, respectively, in the oxidative desulfurization (ODS) process at 60 °C. The catalysts were recycled easily and reused without a significant decrease in their activity. The oxidation of DBT to DBT-sulfone was investigated using gas chromatography, and the formation of DBT-sulfone was confirmed by FT-IR and 1H NMR spectroscopies.
Simple and readily available ketones have been identified to promote the visible-light-promoted aerobic oxidation of sulfides and sulfoxides to sulfones. We report a simple and environmental-friendly oxidation protocol of sulfides to sulfones. Various sulfides were efficiently oxidized into sulfones with O2 as sustainable terminate oxidant, readily available thioxanthone as the photocatalyst and 3-pentanone (DEK) as the solvent. The protocol tolerates diverse functional groups, including halogens, ketone, ester, cyano, heterocycle and even cyclopropyl groups. The detection of the aerobic oxidation reaction in DEK by GC and HRMS disclosed that the key active intermediates were generated.
The oxidation of the sulfide function promoted by a variety of vanadium compounds has been largely explored, whereas the use of homogeneous catalytic systems based on the heavier group 5 metals remains less explored. We report the use of easily available niobium and tantalum carbamates, i.e. [M(O2CNMe2)5] (M = Nb, 1; M = Ta, 2), [Nb(O2CNMe2)4], 3, [NbO(O2CNEt2)3], 4, and [NbCl3(O2CNEt2)2], 5, as effective catalysts for the conversion of a series of alkyl aryl and aromatic sulfides into the corresponding sulfones. NMR investigations on the performant niobium catalyst 4 unexpectedly revealed the substantial stability of this compound in the protic catalytic environment, and a plausible catalytic cycle was obtained by DFT studies. The two active catalytic species, i.e. 4 and its minor mono-methoxide derivative, presumably interconvert to each other exploiting the versatile coordination of the carbamato ligand.
New oxovanadium and dioxomolybdenum Schiff base complexes, [VO(L)(OCH3)] n and [MoO2(L)(CH3OH)], were synthesized by treating an ONO donor Schiff base (H2L) derived by condensation of 3-ethoxysalicylaldehyde and nicotinic hydrazide with oxo and dioxo acetylacetonate salts of vanadium and molybdenum (VO(acac)2 and MoO2(acac)2), respectively. The synthesized ligand and complexes were characterized by FTIR, multinuclear (1H, 13C) NMR, elemental and single crystal X-ray diffraction analysis. In both complexes, the geometry around the central metal ions was distorted octahedral as revealed by diffraction studies. Theoretical calculations of the synthesized compounds were carried out by DFT at B3LYP/Def2-TZVP level of theory, which showed good correlation with the experimental results. Moreover, the catalytic efficiency of both complexes was investigated by oxidizing aryl and alkyl sulfides in the presence of 30% H2O2 in ethanol.
A new ONO-tridentate Schiff base ligand (H2L) derived by the condensation of nicotinic hydrazide with 5-chlorosalicylaldehyde has been prepared and characterized by combustion analysis (CHN), FT-IR and multinuclear (1H and 13C) NMR spectroscopy. The crystalline nature and molecular structure of the ligand were confirmed by single-crystal X-ray diffraction analysis. Furthermore, the optimized structural parameters of the four possible configurations of the ligand including Z and E stereoisomers each containing two tautomeric forms (enol and keto) have also been investigated. The theoretical parameters were calculated by performing the DFT method using the B3LYP/Def2-TZVP level of theory. In addition to this, dioxomolybdenum(VI) (MoO2L) and oxovanadium(V) (VOL) complexes with the entitled Schiff base ligand have also been prepared and characterized by different techniques. Then, the catalytic efficiencies of synthesized VOL and MoO2L complexes were also explored for the oxidation of sulfides using 30% aqueous H2O2 as a source of oxygen. These homogeneous catalysts showed excellent catalytic activities in the oxidation of both aromatic and aliphatic sulfides.
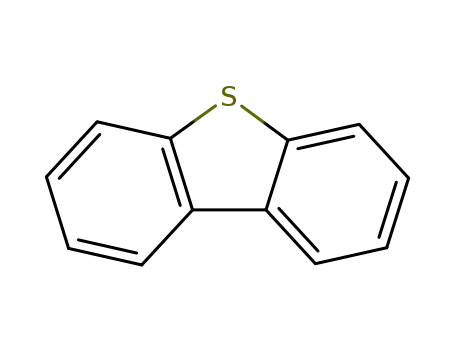
dibenzothiophene

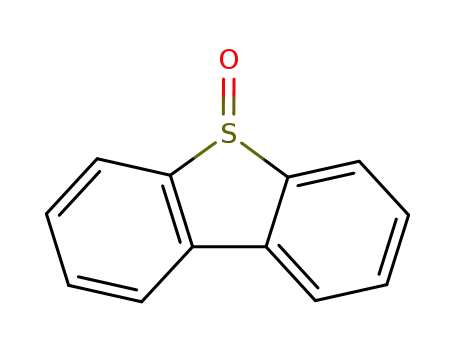
Dibenzothiophene sulfoxide

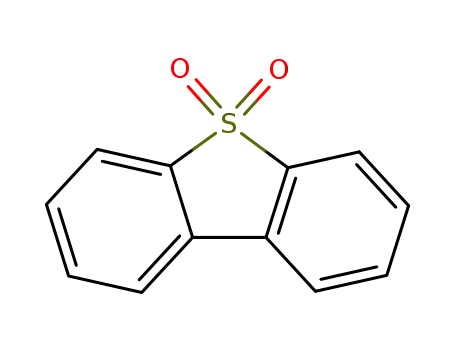
dibenzothiophene sulfone
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; C15H30N5O10Ta;
In
methanol; water;
at 45 ℃;
for 5h;
Catalytic behavior;
Inert atmosphere;
Sealed tube;
|
10% 88% |
|
With
sodium hypochlorite pentahydrate; tetra(n-butyl)ammonium hydrogensulfate;
In
dichloromethane; water; acetonitrile;
at 20 ℃;
for 0.4h;
Green chemistry;
|
86% 6% |
|
With
peroxygenase; dihydrogen peroxide;
In
acetonitrile;
at 20 ℃;
for 0.416667h;
pH=5;
Concentration;
Enzymatic reaction;
|
86% 7.2% |
|
With
tert.-butylhydroperoxide;
In
octane;
at 59.84 ℃;
for 0.5h;
Reagent/catalyst;
Catalytic behavior;
|
18.8% 81.2% |
|
With
tert.-butylhydroperoxide; [Mo2(O)4{[2,2'-(1,3-phenylene)bis(4,5-dihydrooxazole-4,2-diyl)]dimethanol}(acac)2];
In
1,2-dichloro-ethane;
for 1.5h;
Reflux;
|
80% 20% |
|
With
aluminium trichloride; 3-carboxypyridinium chlorochromate;
In
acetonitrile;
for 0.0666667h;
microwave irradiation;
|
75% |
|
With
tert.-butylhydroperoxide; [Mo2(O)4{[2,2'-(1,3-phenylene)bis(4,5-dihydrooxazole-4,2-diyl)]dimethanol}(acac)2];
In
1,2-dichloro-ethane;
for 0.75h;
Reflux;
|
75% 25% |
|
With
iodosylbenzene;
In
ethanol;
at 20 ℃;
Inert atmosphere;
|
30.6% 63% |
|
With
borax; dihydrogen peroxide; sodium hydroxide;
In
methanol; water;
at 20 ℃;
for 10h;
pH=6 - 7;
|
55% 42% |
|
With
bis-[(trifluoroacetoxy)iodo]benzene;
In
chloroform;
at 20 ℃;
for 120h;
|
5% 50% |
|
With
sodium hydroxide; dihydrogen peroxide; N-tosylimidazole;
In
tert-butyl alcohol;
Ambient temperature;
|
8% 50% |
|
With
tert.-butylhydroperoxide;
In
octane;
at 59.84 ℃;
for 0.5h;
Catalytic behavior;
|
50.3% 10.4% |
|
With
water; dihydrogen peroxide; C16H17MoN3O6;
In
ethanol;
|
50% 50% |
|
With
dihydrogen peroxide; C14H12ClMoN3O5;
In
ethanol; water;
for 3.5h;
Reflux;
|
50% 50% |
|
With
tert.-butylhydroperoxide;
In
chloroform;
Product distribution;
|
|
|
With
9,10-Dicyanoanthracene; oxygen;
In
acetonitrile;
Rate constant;
Kinetics;
Irradiation;
|
|
|
With
o-iodosobenzoic acid;
In
water; acetic acid;
for 2h;
Title compound not separated from byproducts;
|
|
|
With
caesium fluoroxysulphate;
In
acetonitrile;
for 1h;
Ambient temperature;
|
78.4 mg 86.0 mg |
|
With
sulfuric acid; dihydrogen peroxide; acetic acid;
In
water;
at 50 ℃;
|
|
|
With
oxygen; isobutyraldehyde;
[C18H37N(CH3)3]5[PV2Mo10O40];
In
acetonitrile;
at 60 ℃;
for 2h;
|
|
|
With
dihydrogen peroxide;
In
methanol;
at 20 ℃;
for 12h;
chemoselective reaction;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 2.16667h;
|
|
|
With
oxoiron(IV) (N,N-bis-(2-pyridylmethyl)bis(2-pyridyl)methylamine)(2+);
In
acetonitrile;
at 24.84 ℃;
Kinetics;
|
|
|
With
C16H30O37Si3W10(4-)*3C16H36N(1+)*H(1+); dihydrogen peroxide;
In
octane; acetonitrile;
at 60 ℃;
for 2h;
Reagent/catalyst;
Time;
Kinetics;
|
|
|
With
(η5-C5H5)Mo(CO)3C2Ph; dihydrogen peroxide;
In
acetonitrile;
at 100 ℃;
for 10h;
Inert atmosphere;
Schlenk technique;
|
|
|
With
formic acid; dihydrogen peroxide;
In
water; toluene;
at 25 ℃;
for 3h;
pH=4.0;
|
|
|
With
bis(di-n-decyldimethylammonium) tungstate; dimethyl-di-n-octylammonium hydrogenosulfate; dimethyl-di-n-octylammonium dihydrogenophosphate; dihydrogen peroxide;
In
water;
at 25 ℃;
for 0.25h;
Concentration;
|
|
|
With
dihydrogen peroxide;
In
water;
at 25 ℃;
for 2h;
Overall yield = 88 %Chromat.;
Green chemistry;
|
|
|
With
sodium phosphotungstate; dihydrogen peroxide; fipronilβ-cyclodextrin;
In
n-heptane; water;
at 65 ℃;
for 0.5h;
Catalytic behavior;
Schlenk technique;
Inert atmosphere;
Green chemistry;
|
|
|
With
α-manganese oxide;
In
octane;
at 25 ℃;
for 0.5h;
Reagent/catalyst;
Temperature;
Catalytic behavior;
Green chemistry;
|
|
|
With
[MoO(O2)2(5-methylpyrazole)2]; dihydrogen peroxide;
In
water;
at 20 ℃;
for 1h;
Reagent/catalyst;
Catalytic behavior;
Ionic liquid;
|
|
|
With
[FeIII2(N,N,N',N'-tetrakis(2-pyridylmethyl)-1,3-diaminopropan-2-ol)(CH3O)(CH3OH)3](ClO4)4; dihydrogen peroxide;
In
acetonitrile;
at 20 ℃;
Reagent/catalyst;
Catalytic behavior;
|
|
|
With
[FeIII2(N,N,N',N'-tetrakis(2-pyridylmethyl)-1,3-diaminopropan-2-ol)(CH3O)(CH3OH)3](ClO4)4; dihydrogen peroxide;
In
acetonitrile;
at 20 ℃;
Reagent/catalyst;
|
|
|
With
dihydrogen peroxide; toluene-4-sulfonic acid;
In
water;
at 60 ℃;
for 24h;
Reagent/catalyst;
Overall yield = 25.5 %Chromat.;
Catalytic behavior;
|
16.7 %Chromat. 8.8 %Chromat. |
|
With
phthalic anhydride; urea hydrogen peroxide adduct;
In
tetrahydrofuran;
at 39.84 ℃;
Reagent/catalyst;
Solvent;
|
|
|
With
[V2(O)4(H2O)4BOX]; dihydrogen peroxide;
In
ethanol;
for 0.75h;
chemoselective reaction;
Catalytic behavior;
Reflux;
|
90%Chromat. 10 %Chromat. |
|
With
dihydrogen peroxide;
In
acetonitrile;
at 65 ℃;
for 0.25h;
Temperature;
|
|
|
With
tert.-butylhydroperoxide;
In
octane;
at 90 ℃;
for 3h;
Reagent/catalyst;
Temperature;
|
|
|
With
dihydrogen peroxide;
In
acetonitrile;
at 20 ℃;
for 5h;
pH=3;
pH-value;
Kinetics;
UV-irradiation;
|
|
|
With
dihydrogen peroxide;
In
ethanol; n-heptane; water;
at 60 ℃;
for 2h;
Time;
|
|
|
With
H3N*12H(1+)*[Mo36(NO)4O108(H2O)16](12-)*30.84H2O; urea hydrogen peroxide adduct;
In
methanol; dichloromethane;
at 20 ℃;
for 0.5h;
Catalytic behavior;
Green chemistry;
|
|
|
With
tert.-butylhydroperoxide; 2.5Co(2+)*4C12H12N6*6H2O*BO40W12(5-);
In
dichloromethane;
at 50 ℃;
Reagent/catalyst;
|
|
|
With
tungsten loaded mesoporous nitrogen rich carbon nanomaterial;
In
acetonitrile;
at 100 ℃;
for 2h;
Reagent/catalyst;
Concentration;
Temperature;
chemoselective reaction;
|
|
|
With
Peroxyformic acid; tetrabutylammomium bromide;
In
toluene;
at 39.84 ℃;
Temperature;
Kinetics;
Sonication;
|
|
|
With
iodosylbenzene; C22H24FeN5O2(2+)*2ClO4(1-);
In
acetonitrile;
at 50 ℃;
for 5h;
Catalytic behavior;
Inert atmosphere;
|
|
|
With
dihydrogen peroxide;
In
acetonitrile;
at 25 ℃;
for 0.5h;
Reagent/catalyst;
Catalytic behavior;
Green chemistry;
|
|
|
With
dihydrogen peroxide;
In
methanol; water;
at 40 ℃;
|
|
|
With
tert.-butylhydroperoxide; [Co(4,4’-bis(1,2,4-triazol-1-ylmethyl)biphenyl)3][HPMo12O40]·24H2O;
In
dichloromethane;
at 50 ℃;
for 0.25h;
Reagent/catalyst;
|
|
|
With
[Cu(2,2′-dipyridine amine)(acetylacetone anion)(MeOH)]2[VIV2VV4O11(OMe)8]; dihydrogen peroxide;
In
water; acetonitrile; decalin;
at 40 ℃;
for 6h;
Time;
Reagent/catalyst;
Catalytic behavior;
|
|
|
With
tert.-butylhydroperoxide; 0.5C140H128N24O8*3C3H7NO*5H2O*2Co(2+)*V4O12(4-);
In
dichloromethane;
at 50 ℃;
for 12h;
Overall yield = 93 %;
|
|
|
With
dihydrogen peroxide;
In
1,2-dichloro-benzene; acetonitrile;
at 45 ℃;
for 24h;
Reagent/catalyst;
Kinetics;
|
|
|
With
dihydrogen peroxide; acetic acid;
at 25 ℃;
Kinetics;
|
|
|
dibenzothiophene;
With
C128H96N32Pd4(12+)*Mo8O26(4-);
In
water-d2;
at 20 ℃;
for 3h;
With
tert.-butylhydroperoxide;
In
water-d2;
at 60 ℃;
for 9h;
Reagent/catalyst;
Time;
Catalytic behavior;
|
|
|
With
K6LiH6[Ce4(H2O)14(2,3-pyrazinedicarboxylato)(2,3-pyrazinedicarboxylic acid)As3W29O103]*22H2O; dihydrogen peroxide;
In
ethanol; toluene;
at 25 ℃;
for 1h;
|
|
|
With
oxygen;
In
octane;
Reagent/catalyst;
Irradiation;
|
|
|
dibenzothiophene;
In
hexane;
at 60 ℃;
for 0.0333333h;
Sonication;
With
tert.-butylhydroperoxide;
In
hexane; water;
at 60 ℃;
for 2h;
Reagent/catalyst;
Catalytic behavior;
|
|
|
With
tert.-butylhydroperoxide; dihydrogen peroxide;
In
octane;
at 60 ℃;
for 2h;
Reagent/catalyst;
Catalytic behavior;
|
|
|
With
tin(IV) oxide; dihydrogen peroxide;
In
octane;
at 59.84 ℃;
for 2h;
Temperature;
Reagent/catalyst;
Time;
Catalytic behavior;
Activation energy;
|
|
|
With
tert.-butylhydroperoxide;
In
toluene;
at 80 ℃;
Reagent/catalyst;
Temperature;
|
|
|
With
tert.-butylhydroperoxide; Mo12O40P(3-)*2C60H52N4O12S4*2Co(2+)*Cl(1-)*6C2H3N;
In
dichloromethane;
at 50 ℃;
for 10h;
Reagent/catalyst;
Overall yield = > 99 percent;
|
|
|
With
tert.-butylhydroperoxide; Mo12O40P(3-)*4Ag(1+)*2C64H80N8O4S8*HO(1-);
In
dichloromethane;
at 40 ℃;
for 12h;
|
|
|
With
C60H44CoN12O8S4; dihydrogen peroxide;
In
dodecane; acetonitrile;
at 40 ℃;
for 2h;
under 760.051 Torr;
Temperature;
Time;
Reagent/catalyst;
Kinetics;
|
|
|
With
dihydrogen peroxide;
In
acetonitrile; decalin;
at 70 ℃;
for 1h;
Reagent/catalyst;
Temperature;
Kinetics;
Catalytic behavior;
|
|
|
With
tert.-butylhydroperoxide; 3Ag(1+)*C2H3N*4H2O*Mo12O40P(3-)*2C68H76N4O4;
In
methanol;
at 50 ℃;
for 4h;
|
|
|
With
C6O61P2Re2W17(10-)*6C4H12N(1+)*4H(1+); dihydrogen peroxide;
In
acetonitrile;
at 60 ℃;
|
|
|
With
dihydrogen peroxide; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate;
In
octane;
at 60 ℃;
for 0.416667h;
Temperature;
Reagent/catalyst;
Catalytic behavior;
Kinetics;
|
|
|
With
tert.-butylhydroperoxide; C60H56MnN4O16S4*Mo6O19*0.5C2H6O*H2O;
In
dichloromethane;
at 40 ℃;
for 5h;
Reagent/catalyst;
|
|
|
With
9,10-Dicyanoanthracene; oxygen;
In
acetonitrile;
at 20 ℃;
for 0.166667h;
under 6750.68 Torr;
Irradiation;
Flow reactor;
|
|
|
With
tert.-butylhydroperoxide; [Zn1.5(2,6-bis(2'-pyridyl)-4-hydroxypyridine)3]*(PMo12O40)*CH3OH*2H2O;
In
dichloromethane;
at 50 ℃;
for 12h;
Time;
Catalytic behavior;
|
|
|
With
tert.-butylhydroperoxide; (NH2Me2)12[(V5O9Cl)6(biphenyl 3,4',5-tricarboxylate)8]*[MeOH]7;
In
methanol;
at 50 ℃;
for 6h;
Temperature;
Catalytic behavior;
|
|
|
With
tert.-butylhydroperoxide; C56H72N16O4S8*3Ag(1+)*Mo12O40P(3-);
In
biphenyl; dichloromethane;
at 25 ℃;
for 12h;
Reagent/catalyst;
|
|
|
With
64H2O*21Na(1+)*10H(1+)*4CO3(2-)*[(AsO4){Ni8(OH)6(SiW9O34)2}2](23-);
at 70 ℃;
for 2h;
|
|
|
With
Cumene hydroperoxide;
at 100 ℃;
for 4h;
Reagent/catalyst;
Temperature;
|
|
|
With
dihydrogen peroxide;
In
octane; acetonitrile;
at 30 ℃;
for 0.416667h;
Reagent/catalyst;
Temperature;
Catalytic behavior;
Kinetics;
|
|
|
With
dihydrogen peroxide; DyH8O80P2W20Zn4(11-)*23H2O*11K(1+);
In
water; acetonitrile;
at 40 ℃;
for 1h;
|
|
|
With
tungsten oxide; dihydrogen peroxide;
In
d(4)-methanol;
for 0.5h;
Time;
|
|
|
at 40 ℃;
for 1.25h;
Green chemistry;
|

Dibenzothiophene sulfoxide


dibenzothiophene sulfone
| Conditions | Yield |
|---|---|
|
With
water; chlorine;
In
acetic acid;
at 25 ℃;
Mechanism;
Rate constant;
|
|
|
With
[bis(acetoxy)iodo]benzene; water;
perchloric acid;
In
acetic acid;
at 49.9 ℃;
Thermodynamic data;
Mechanism;
Rate constant;
ΔH(excit.), ΔS(excit.);
|
|
|
With
molybdenum peroxide hexamethylphosphorylamide;
In
1,2-dichloro-ethane;
at 40 ℃;
Rate constant;
|
|
|
With
water; chlorine;
In
acetic acid;
at 25 ℃;
|
|
|
With
TBA4H2[BW11Mn(H2O)O39]*H2O; dihydrogen peroxide;
In
water; acetonitrile;
at 22 - 24 ℃;
Darkness;
Green chemistry;
|
|
|
Multi-step reaction with 2 steps
1: xenon difluoride; tetrabutyl-ammonium chloride / dichloromethane / 3 h / Inert atmosphere
2: toluene / 0.17 h / -35 °C / Inert atmosphere
With
xenon difluoride; tetrabutyl-ammonium chloride;
In
dichloromethane; toluene;
|
|
|
With
dihydrogen peroxide;
In
2,2,4-trimethylpentane;
at 80 ℃;
for 1.66667h;
Temperature;
|
|
|
With
tert.-butylhydroperoxide; meso-tetrakis(o-chlorophenyl)porphineiron(III) chloride;
In
methanol; water;
at 30 ℃;
for 14h;
Irradiation;
|
55.7 %Chromat. |
|
With
N,N'-bis(3,5-di-tert-butylsalicylidene)ethylenediaminocobalt(II); dihydrogen peroxide;
In
dodecane; acetonitrile;
under 760.051 Torr;
|
|
|
With
dihydrogen peroxide;
In
water; acetonitrile;
at 60 ℃;
under 760.051 Torr;
|
|
|
With
tert.-butylhydroperoxide;
at 60 ℃;
Reagent/catalyst;
Temperature;
Catalytic behavior;
|
n-butyllithium
diethyl ether
thianthrene-5,5,10-trioxide
2-phenylbenzenesulfonyl chloride

dibenzothiophene
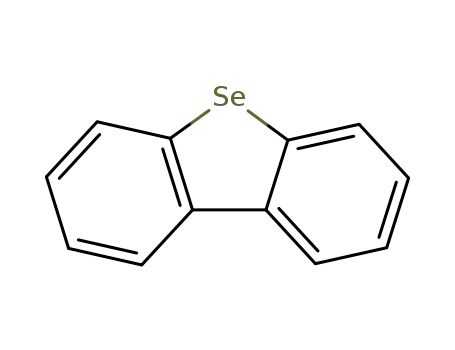
dibenzo[b,d]selenophene
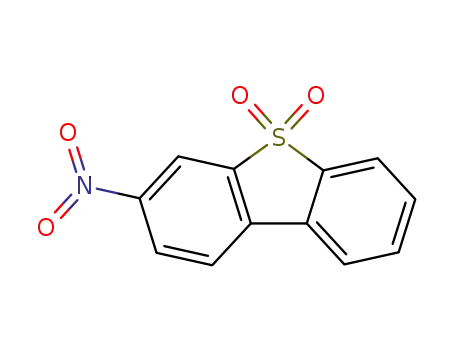
3-nitrodibenzo[b,d]thiophene 5,5-dioxide
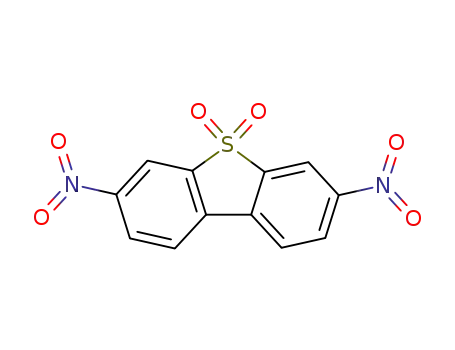
3,7-dinitrodibenzothiophene sulfone