Your Location:Home >Products >Organic phosphines >Other organic phosphines >998-40-3


Product Details
Chemical Properties
Clear liquid
Uses
1,4-addition catalyst; used with disulfides for the thioetherification of alcohols; acylation catalyst; used to prepare active esters.
Uses
suzuki reaction
Uses
Tributylphosphine is usually used as catalyst for Domino reactions of activated conjugated dienes with β,γ-unsaturated-α-ketoesters, [3+2]-Cycloadditions,Umpolung addition reactions,Reductive carbonylation, Allylation reactions.
Synthesis Reference(s)
Tetrahedron Letters, 25, p. 2493, 1984 DOI: 10.1016/S0040-4039(01)81213-2
General Description
Tributylphosphane is a colorless to yellowish liquid with a strong garlic-like odor. Tributylphosphane is insoluble in water. Tributylphosphane is liable to heat and ignite spontaneously in air. If involved in a fire phosphine gas, a highly flammable and toxic gas, will evolve. Tributylphosphane is irritating to mucous membranes.
Air & Water Reactions
Highly flammable. May ignite on contact with air or moist air. Insoluble in water.
Reactivity Profile
Organophosphates, such as Tributylphosphane, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Purification Methods
Fractionally distil it under reduced pressure in an inert atmosphere (N2) through an 8inch gauze-packed column (b 110-111o/10mm) and redistil it in a vacuum, then seal it in thin glass ampoules. It is easily oxidised by air to tri-n-butylphosphine oxide, b 293-296o/745mm. It has a characteristic odour, it is soluble in EtOH, Et2O, and *C6H6 but is insoluble in H2O and less easily oxidised by air than the lower molecular weight phosphines. It forms complexes, e.g. with CS2 (1:1) m 65.5o (from EtOH). [Davies & Jones J Chem Soc 33 1929, Chernick & Skinner J Chem Soc 1401 1956, Beilstein 4 IV 3436.]
InChI:InChI=1/C12H27P/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
The irreversible addition of alkyl radicals to diethyl vinylphosphonate to give the α-phosphorylalkyl radicals (1) has been studied by e.s.r. spectroscopy at 233 K in hydrocarbon solvents.The rate constants for addition (kadd) have been determined relative to those (2kAt) for self-reaction of (1) for a series of addenda.The values of 2kAt have been measured in separate kinetic e.s.r. experiments for (1; R = Me) (2.0 x 1E9 dm3mol-1s-1) and (1; R = But) (5.0 x 1E8 dm3mol-1s-1) and thus absolute values of kadd were obtained.The rate constant kadd increases along the series R. = Me. (2.5 x 1E3 dm3mol-1s-1 ) prim. sec. t. (5.9 x 1E4 dm3mol-1s-1) and this order is interpreted in terms of the over-riding importance of polar effects in determining the size of the barrier for addition of these nucleophilic radicals to the electron deficient alkene.R. + H2C=CHP(O)(OEt)2 --> RCH2C.HP(O)(OEt)2 (1)
The dinuclear phosphido-bridged manganese complex undergoes thermal CO substitution by PnBu3 in decalin to give nBu3)> and nBu3P)(OC)3Mn(μ-PEt2)2Mn(CO)3(PnBu3)>.A kinetic study of the first CO/PnBu3 exchange indicates that all substitutions for the forward and reverse reactions occur via a dissociative mechanism.The rate of CO dissociation is rather low (k = 6.27 * 10-5 s-1 at 130 deg C), mainly as a consequence of a high activation enthalpy (ΔH* = 165 kJ mol-1).The discriminating ability ofthe resulting coordinatively unsaturated intermediate is only slightly in favour of attack of the more basic PnBu3 compared to CO, as indicated by the values of the competition rate ratio nBu3) = 0.36-0.47>.This behaviour is compared with that of the isostructural chromium complex and discussed in the light of the electronic and structural features related to the metal-metal distance in the two complexes (Mn-Mn = 3.675 Angstroem, Cr-Cr = 2.905 Angstroem, for the μ-PMe2 derivatives).
The compound Se2 (1) reacts with primary arsanes and phosphanes, and migration of the carbonylmetal fragment Cp'Mn(CO)2 (Cp'=CH3C5H4) from the selenium to the arsenic or phosphorus center occurs.Products of these reactions are diselena-diarsetanes (4) or diselena-diphosphetanes (5).During the reaction of arsanes with 1, arsinidene complexes (2) are obtained in very good yields.Compounds with a P-Se-P unit (6) can be isolated as by-products of the reaction of phosphanes with 1.
The mechanism of the reduction of phosphine oxides by PhSiH3 was established on the basis of kinetic measurements and Density Functional Theory (DFT) calculations. In particular, it has been proved that the model reaction between tri-nbutylphosphine oxide and phenylsilane occurs via a nonpolar mechanism. The data presented herein allow prediction and verification of the applicability of the new reduction reagents and conditions for industrially attractive processes.
Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]? as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction with the phosphine oxide provides a phosphonium salt, further reduced by the silane to afford the desired phosphine along with siloxanes. (Figure presented.).
Investigations on the boundaries between the neutral and cationic models of (Mesityl)2EX (E = Sb, Bi and X = Cl?, OTf?) have facilitated reversing the Lewis acidity from bismuth to antimony. We use this concept to demonstrate a higher efficiency of (Mesityl)2SbOTf(Mesityl)2BiOTf in the catalytic reduction of phosphine oxides to phosphines. The experiments supported with computations described herein will find use in designing new Lewis acids relevant to catalysis.
The kinetics of quinuclidine displacement of BH3 from a wide range of Lewis base borane adducts have been measured. Parameterization of these rates has enabled the development of a nucleofugality scale (NFB), shown to quantify and predict the leaving group ability of a range of other Lewis bases. Additivity observed across a number of series R′3-nRnX (X = P, N; R′ = aryl, alkyl) has allowed the formulation of related substituent parameters (nfPB, nfAB), providing a means of calculating NFB values for a range of Lewis bases that extends far beyond those experimentally derived. The utility of the nucleofugality parameter is explored by the correlation of the substituent parameter nfPB with the hydrolyses rates of a series of alkyl and aryl MIDA boronates under neutral conditions. This has allowed the identification of MIDA boronates with heteroatoms proximal to the reacting center, showing unusual kinetic lability or stability to hydrolysis.
By introducing trimethylsilyl chloride (TMSCl), the pentavalent phosphoryl P(V) compounds such as triphenylphosphine oxides, secondary phosphine oxides etc., were readily converted to the corresponding R2P(OTMS) intermediates, that can further react efficiently with an electrophile R'X or with a nucleophile R'Li to produce the corresponding trivalent phosphines R2PR’. Chiral phosphines could also be obtained stereospecifically by this strategy.
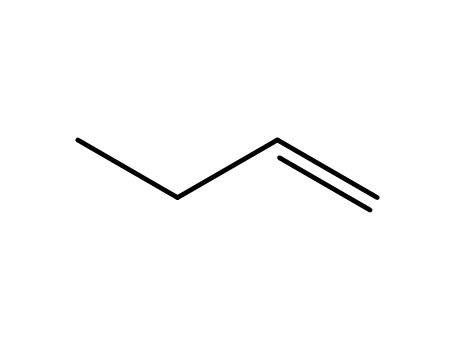
1-butylene

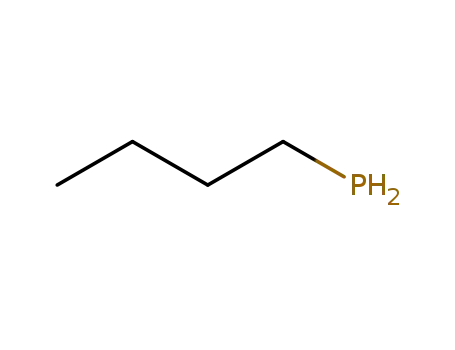
n-butylphosphine


di-n-butylphosphine


tributylphosphine
| Conditions | Yield |
|---|---|
|
at 25 ℃;
UV-Licht.Irradiation;
|

1-butylene

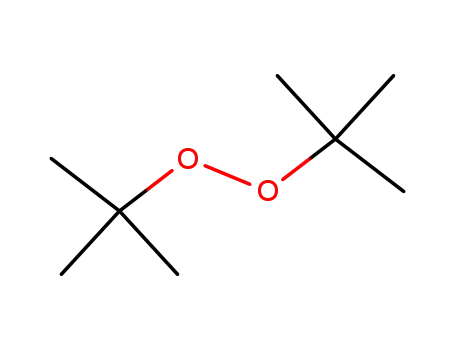
di-tert-butyl peroxide


n-butylphosphine


di-n-butylphosphine


tributylphosphine
| Conditions | Yield |
|---|---|
|
at 122 ℃;
|

1-butylene

di-tert-butyl peroxide
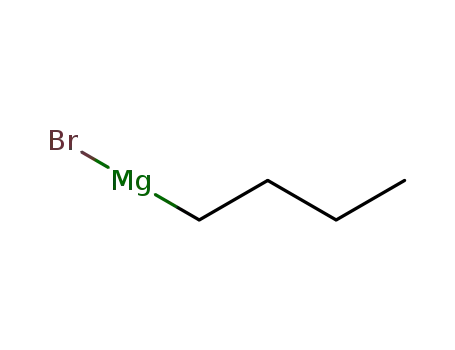
n-butyl magnesium bromide
diethyl ether

tetra-n-butylphosphonium iodide
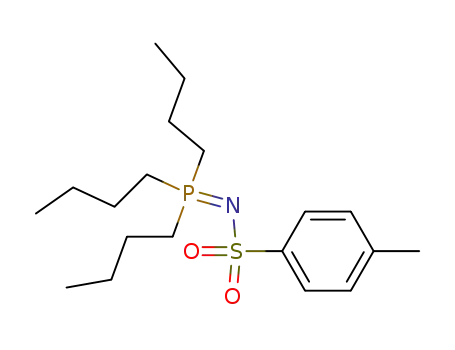
4-methyl-N-(tri-N-butylphosphoranylidene)-benzenesulfonamide
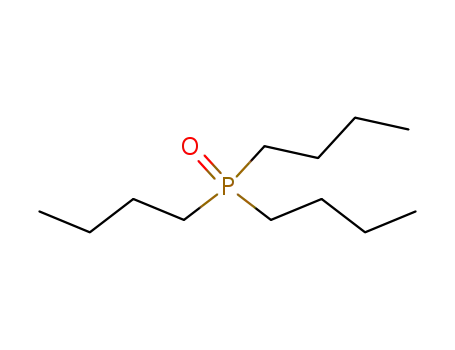
Tributylphosphine oxide
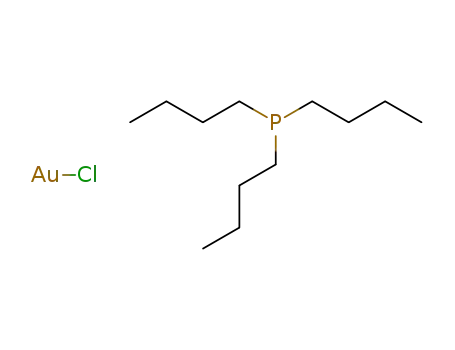
tributyl-phosphine; compound with gold(I)-chloride
CAS:5518-52-5
CAS:20445-88-9