Your Location:Home >Products >Organic phosphines >Phenyl phosphines >5525-40-6
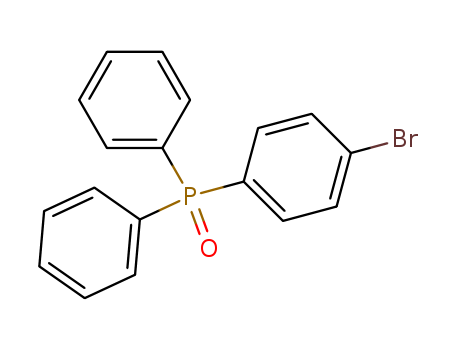

Product Details
InChI:InChI=1/C21H17NO/c23-20-13-7-12-18-21(20)17(15-8-3-1-4-9-15)14-19(22-18)16-10-5-2-6-11-16/h1-6,8-11,14H,7,12-13H2
Phosphorescent and thermally activated delayed fluorescence (TADF) emitters have met a bottleneck due to the efficiency roll-off problem caused by the accumulation of the lowest triplet excited states with a relatively long lifetime. To achieve a high performance electroluminescence (EL) device with a simultaneously high radiative exciton ratio and a low efficiency roll-off in lighting applications at high luminance, we have demonstrated a novel strategy to construct an efficient hot exciton channel by effective spin mixing of the high-lying singlet and the triplet charge transfer (CT) excited states and restriction of the internal conversion (IC) from the high-lying triplet CT state to the lower triplet state. We selected luminous anthracene and electron-withdrawing triphenylphosphine oxide groups with a high triplet energy level to build four moderated D-A structure molecules which possess a high-lying CT hot exciton. Furthermore, the electronic coupling between the D and A unit can be precisely reduced by means of twisted structure regulation. In our series of novel synthesized materials, 9-[4-(diphenyl-phosphinoyl)-2-methyl-phenyl]-anthracene (An9-MePo) achieved optimization of the hot exciton radiation process and restriction of the IC. Both the maximum radiative exciton ratio of 74% and 72%, and the low efficiency roll-off of 10.6% and 15.9% at a luminance of 10000 cd m-2 were achieved in a practical lighting application by the An9-MePo based nondoped and doped EL devices, respectively. Our results provide a novel approach to the fabrication of an efficient hot exciton channel, proving the great potential of this EL molecule design strategy for practical lighting applications.
The heterotopic ligand 1-[4-(4-diphenylphosphanylphenyl)phenylimino]-2-[3,5-bis(trifluoromethyl)phenylimino]acenaphthene, PN2, was prepared and characterized by NMR spectroscopic and MS methods. Depending on the size and the electronic properties of the metal reagent M, the two binding sites of the new ligand show selective coordination. At 1:1 molar ratio of PN2 and M, the sterically more demanding [Pd(dppe)]2+ and [Pd(dppp)]2+ cations coordinate only to the chelating nitrogen atoms while the less bulky [Pd(dppm)]2+ binds both to the imino and the phosphane donor groups. A 2:1:2 mixture of PN2, [Pd(dppm)(OTf)2] and [Pd(dppe)(OTf)2] results in the formation of a complex in which the [Pd(dppm)]2+ unit selectively coordinates to the terminal P atoms while the more voluminous dppe counterpart resides at the imino nitrogens. On the contrary, the [Pd(dppm)]2+ complex selectively binds to the chelating nitrogen atoms if the Ph2P group is ligated to Au+ center. In the interaction of [Au(PN2)2]PF6 with Ag(OTf), the silver ions do not replace Au+ in the P-Au bonds but occupy the chelating position.
Tertiary phosphines remain widely utilized in synthesis, most notably as supporting ligands in metal complexes. A series of triarylphosphines bearing one to three hexa-peri-hexabenzocoronene (HBC) substituents has been prepared by an efficient divergent route. These "superphenylphosphines", P{HBC(t-Bu)5}nPh3-n (n = 1-3), form the palladium complexes PdCl2L2 and Pd2Cl4L2 where the isomer distribution in solution is dependent on the number of HBC substituents. The crystalline structures of five complexes all show intramolecular π-stacking between HBC-phosphines to form a supramolecular bidentate-like ligand that distorts the metal coordination geometry. When n = 2 or 3, the additional HBC substituents engage in intermolecular π-stacking to assemble the complexes into continuous ribbons or sheets. The phosphines adopt HBC's characteristics including strong optical absorption, green emission, and redox activity.
A simple azine-linked covalent organic framework (COF) with high thermal and chemical stabilities has been prepared by using deep eutectic solvent (DES) as green media. The as-synthesized COF was employed as heterogeneous ligand for immobilization of PdII. The obtained Pd-supported COF nanoparticles catalyst (defined as Pd/TFPT-Azine-COF) was found to be an efficient heterogeneous catalyst for the Hirao reaction of aryl halides and dialkyl phosphites or diphenylphosphine oxide with excellent recyclability, reusability, and retention of crystallinity.
The invention relates to the technical field of photoelectric display devices, in particular to an aggregation-induced light emission material and a preparation method thereof. The invention provides an aggregation-induced light emission material. The structural formula of the material is as shown in a formula (I). The preparation method of the aggregation-induced light emission material comprises the following step of: carrying out Suzuki coupling reaction on a compound as shown in a formula (II) and (4-bromophenyl) diphenyl phosphine oxide to prepare the compound as shown in the formula (I). With the aggregation-induced light emission material and the preparation method thereof of the invention adopted, the technical problems that an existing organic light-emitting material is poor in performance and easily generates an aggregation quenching light-emitting effect can be solved.
The invention relates to a compound for organic luminescence, wherein the structure of the compound is shown as a formula (I), R1-R3 are independently selected from hydrogen, a substituted or unsubstituted aryl group, a substituted or unsubstituted heteroaryl group and a substituted or unsubstituted alkyl group respectively, L is selected from substituted or unsubstituted phenyl and substituted orunsubstituted heteroaryl, and Ar1 and Ar2 are respectively and independently selected from substituted or unsubstituted phenyl, naphthyl and anthryl. The compound for organic luminescence can be usedas an electron transport material, and has high stability, high charge transfer capability and high glass transition temperature.
The invention belongs to the technical field of organic electron transport materials, and discloses an asymmetrically substituted anthryl derivative as well as preparation and application thereof. Theasymmetrically substituted anthryl derivative is one of the following compounds (as shown in the specification). The invention also discloses a preparation method of the asymmetrically substituted anthryl derivative. An organic electron transport material comprises more than one of the asymmetric substituted anthryl derivatives. An n-type doped electron transport layer is obtained by carrying outn-type doping on the organic electron transport material. The organic electron transport material has the advantages of good solubility, high thermal decomposition temperature, high glass transitiontemperature and the like, and the electron transport layer formed through n-type doping is applied to electroluminescent devices, especially red phosphorescent devices, and has high stability.
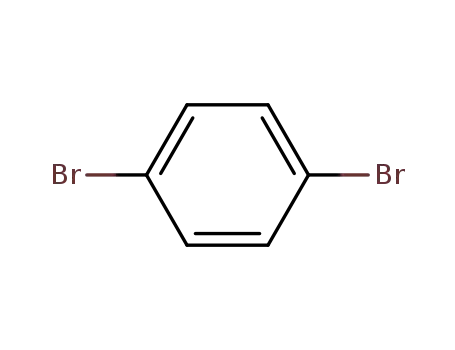
1.4-dibromobenzene

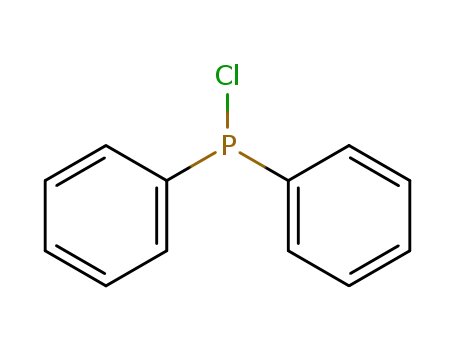
chloro-diphenylphosphine

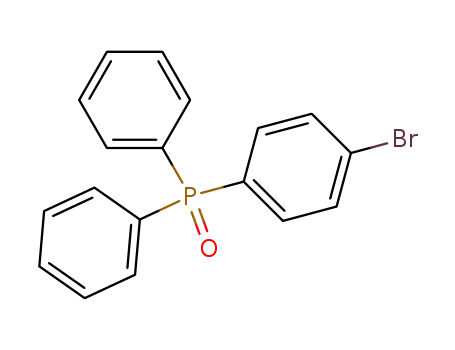
(4-bromophenyl)diphenylphosphine oxide

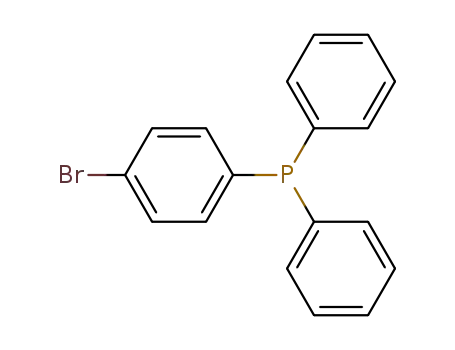
(4-bromophenyl)diphenylphosphane
| Conditions | Yield |
|---|---|
|
With
magnesium;
In
diethyl ether;
at -10 ℃;
for 1h;
|
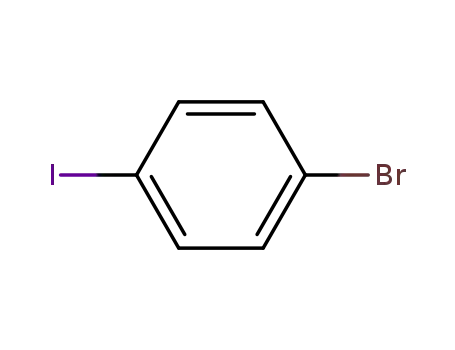
1,4-bromoiodobenzene


chloro-diphenylphosphine


(4-bromophenyl)diphenylphosphine oxide
| Conditions | Yield |
|---|---|
|
1,4-bromoiodobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
chloro-diphenylphosphine;
In
tetrahydrofuran;
at -78 ℃;
for 2h;
|
71.4% |
|
1,4-bromoiodobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
chloro-diphenylphosphine;
In
tetrahydrofuran;
for 2h;
Inert atmosphere;
With
dihydrogen peroxide;
In
dichloromethane; water;
at 25 ℃;
for 18h;
|
71.4% |
|
1,4-bromoiodobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
chloro-diphenylphosphine;
In
tetrahydrofuran;
for 2h;
Inert atmosphere;
With
dihydrogen peroxide;
at 0 - 20 ℃;
for 18h;
Inert atmosphere;
|
71.4% |
|
1,4-bromoiodobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
chloro-diphenylphosphine;
In
tetrahydrofuran; water;
at -78 - 20 ℃;
for 2h;
Inert atmosphere;
With
dihydrogen peroxide;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
for 18h;
Inert atmosphere;
|
71.4% |
|
1,4-bromoiodobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.75h;
Inert atmosphere;
chloro-diphenylphosphine;
In
tetrahydrofuran; hexane;
at 20 ℃;
With
dihydrogen peroxide;
In
tetrahydrofuran;
at 20 ℃;
for 2h;
|
2.25 g |
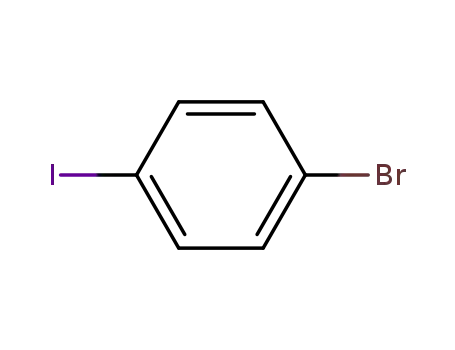
1,4-bromoiodobenzene
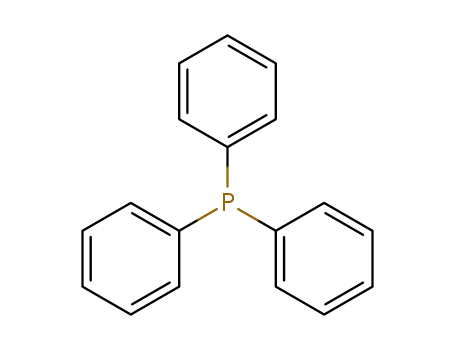
triphenylphosphine
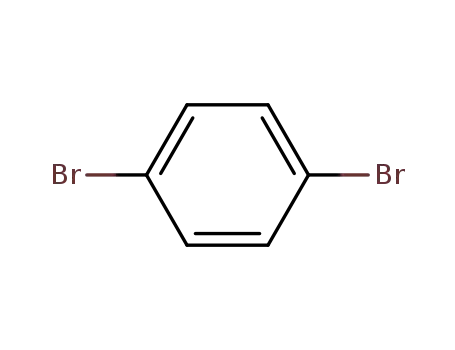
1.4-dibromobenzene
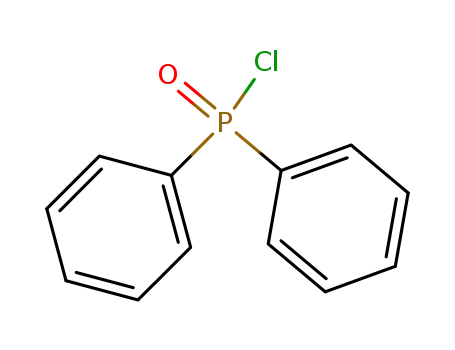
Diphenylphosphinic chloride
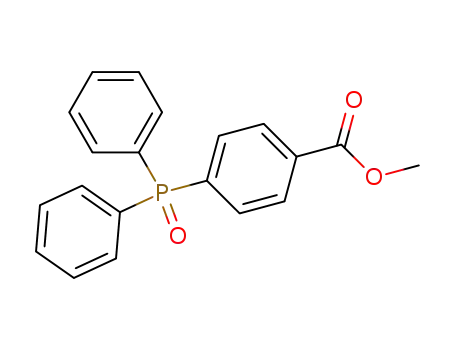
(4-methoxycarbonylphenyl)diphenylphosphine oxide
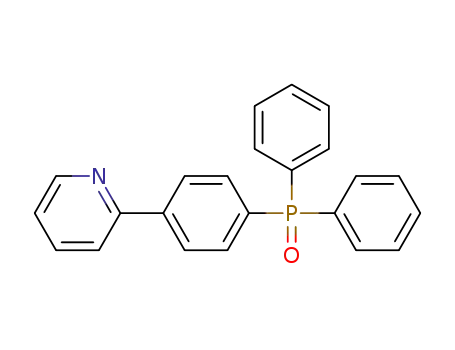
2-[(4-diphenylphosphoryl)phenyl]pyridine
1-(4-diphenylphosphine oxide)phenyl-2-N,N-dimethylaminomethylferrocene
CAS:69227-47-0
CAS:240417-00-9