Your Location:Home >Products >Organic phosphines >Phenyl phosphines >4559-70-0
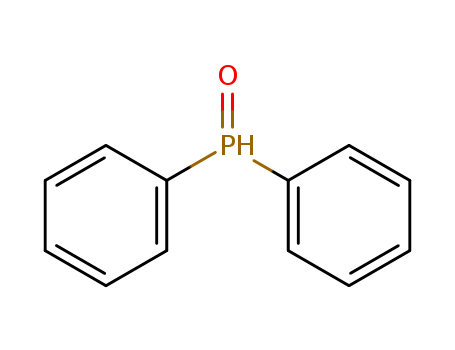

Product Details
Chemical Properties
Yellow to light orange crystals
Uses
suzuki reaction
Uses
Used in the preparation of triarylphosphine oxides, and as asymmetric catalysts, preparation of alkenyldiphenylphosphine oxides, and Horner-Wittig reagents. It is a ligand for Buchwald-Hartwig Cross Coupling Reaction, Hydrophosphonylations and Suzuki-Miyaura Coupling.
Synthesis Reference(s)
Journal of the American Chemical Society, 45, p. 165, 1923 DOI: 10.1021/ja01654a024
InChI:InChI=1/C12H10OP/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H/q+1
γ-Ketophosphine chalcogenides, precursors for plethora of novel functionalized phosphine chalcogenides and phosphines, are synthesized by chemo-and regioselective addition of secondary phosphine chalcogenides to β,γ-ethylenic ketones under catalyst-and so
A dynamic axially chiral BIPHEP-ligand with 3,5-dichlorobenzoyl amide selector units for non-covalent binding of phenylalanine derivatives has been developed. Interaction studies in solution were performed with rhodium(i) complexes under exclusion of the metal being involved in binding. (Rax, SPhe) and (Sax, SPhe) adducts were observed as significantly separated species in NMR spectroscopy.
Highly functionalized benzo[b]phosphole oxides were synthesized from reactions of arylphosphine oxides with alkynes under photocatalytic conditions by using eosin Y as the catalyst and N-ethoxy-2-methylpyridinium tetrafluoroborate as the oxidant. The reac
Cycloadditions of 2-diphenylphosphinoyl-2-methyl-3,4-dihydro-2H-pyrrole N-oxide (DPhPMPO), 3,4-dihydro-2H-pyrrole N-oxide having a diphenylphosphinoyl group at the C2 position with thioketones afforded the corresponding 1,4,2-oxathiazolidines. Dissociation constants of these 5-membered ring products were determined. The cycloadducts were stabilized by the diphenylphosphinoyl group. The reaction of DPhPMPO with di-tert-butyl selenoketone gave the corresponding selenoamide under microwave irradiation. The formation of the selenoamide indicated that the cycloaddition of DPhPMPO with the selenoketone analogue also proceeded through the formation of the corresponding 5-membered ring product.
Extraction behavior of some selected actinides like U(VI), Th(IV), and Am(III) was investigated with three different H-phosphine oxides, viz. diphenyl hydrogen phosphine oxide (DPhPO), dihexyl hydrogen phosphine oxide (DHePO) and diphenyl phosphite (DPP).
This study details the oxidation of α-hydroxy(2,4,6-trimethylbenzyl)diphenylphosphine oxide (α-HDPO) to diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO) by choosing the proper MnO2 as oxidant. In addition, the equilibrium of α-HDPO and 2,4
(l-Naphthoyl)diphenylphosphine oxide (1) was synthesized and characterized and its photochemistry investigated using emission spectroscopy, pulse radiolysis, and nanosecond laser flash photolysis. Fluorescence quantum yields are low in aprotic polar and nonpolar solvents. In methanol, as a result of hydrogen-bonding, change of S1 from an n,π* state to a π,π* state leads to the decrease in the rate constant for intersystem crossing and results, finally, in an increase in fluorescence. Preferential solvation was evaluated using the ET indicator "Reichardt's dye" (RD). ETN values were determined by gradually increasing the concentrations of methanol in methanol/acetonitrile mixtures. Fluorescence quantum yields correlate with the ETN values. Photolysis of 1 yields diphenyl[l-naphthoyl)oxy]phosphine (6), formed mainly via cage recombination of radicals. No radicals were detected by either nanosecond laser flash photolysis or pulse radiolysis of 1 in aprotic solvents. However, photolysis in methanol yields radicals when 1 is excited at 266 nm. The phosphinoyl radical can be quenched by either methyl methacrylate (MMA) or oxygen (kq = 5.0 × 107 and 5.3 × 108 M-1 s-1, respectively). Such radical generation most likely results from the singlet excited state.
[Figure not available: see fulltext.] As a result of the implementation of two approaches, the Pudovik reaction (the reaction of macrocyclic azomethines with secondary phosphine oxides) and the Kabachnik–Fields reaction (three-component one-pot process involving dialdehydes, diamines, and secondary phosphine oxides), novel N,O-containing macrocyclic ligands with phosphine oxide groups were first obtained. The synthesized macrocycles are a kind of α-aminophosphoryl compounds that can be used in the synthesis of supramolecular systems.
The photochemistry of a series of bis(acyl)phosphine oxides and the rate constants of the reactions of their phosphorus radicals with n-butylacrylate, thiophenol, bromotrichloromethane, oxygen, and methyl viologen have been investigated by laser flash pho
-
Transition-metal-catalyzed branched and enantioselective allylic substitution of monosubstituted precursors with carbon, nitrogen, oxygen, sulfur, and fluoride nucleophiles has been well-established. However, such a selective carbon-phosphorus bond formation has not been realized probably due to the catalyst deactivation by the strong coordinating nature of phosphinylating reagents. Herein, we report a Rh-catalyzed highly regio- and enantioselective synthesis of allylic phosphine oxides in the presence of a chiral bisoxazoline-phosphine ligand. The application of α-hydroxylalkylphosphine oxides to keep the low concentration of the secondary phosphine oxides is essential for the high yields. The addition of diphenyl phosphoric acid was found to not only activate allylic alcohols but also accelerate the carbon-phosphorus bond formation.
We describe here the coupling to transform aryl phosphine derivatives by the cleavage of unactivated C(aryl)-P bonds with chromium catalysis, allowing us to achieve the reaction with alkyl bromides and arylmagnesium reagents under mild conditions. Mechani
We herein report a direct electrochemical dehydrogenative C–H phosphonylation of thiazoles derivatives with H2 evolution. Employing electricity as the green and sole oxidant, cheap metal as electrode, the anodic oxidation together with cathodic hydrogen evolution process provides a green and efficient strategy for C–H phosphonylation. A diverse range of phosphorus products were constructed under external metal and oxidant-free conditions at ambient temperature, featuring atom economy, simple operation and wide reaction scope.
An efficient protocol for concurrent tandem halogen exchange/C?P cross-coupling of cycloalkenyl bromides and secondary phosphine oxides has been developed. The catalytic system is based on cheap and air-stable copper(I) iodide as the precatalyst, commercially available N,N’-dimethylethylenediamine as the ligand, and Cs2CO3 or K2CO3 as the base. The use of sodium iodide as an additive reduces the excessive use of organic bromides to near-stoichiometric by promoting the in situ transformation to the corresponding iodides. Diarylphosphine oxides undergo cycloalkenylation with 35–99 % yields and dicyclohexylphosphine oxide with 30–53 % yields. In the case of acyclic alkenyl bromides the cross-coupling products undergo conjugate addition of diphenylphosphine oxide and satisfying yields are observed only for internal olefins. In the case of aryl bromides satisfying yields (43–72 %) are observed only for sterically unhindered arenes or arenes possessing an ortho-directing group. Cycloalkenylphosphine oxides prepared in the cross-coupling reaction undergo base-catalyzed and base-promoted conjugate addition to give bis(phosphinoyl)cycloalkanes.
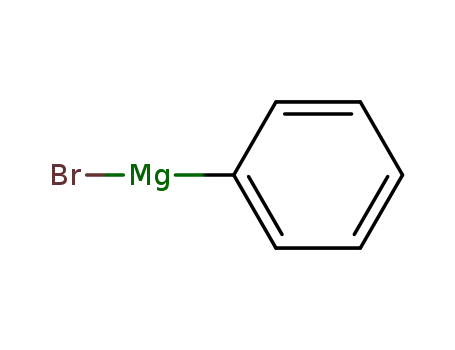
phenylmagnesium bromide

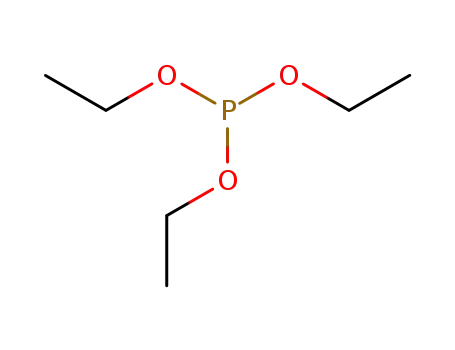
triethyl phosphite

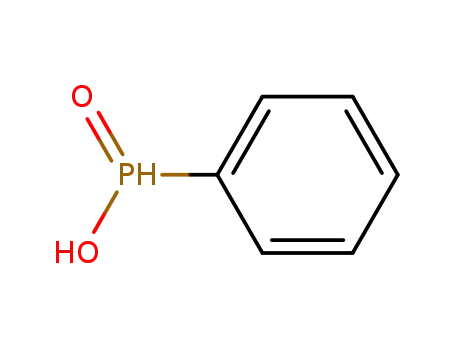
phenylphosphinic acid

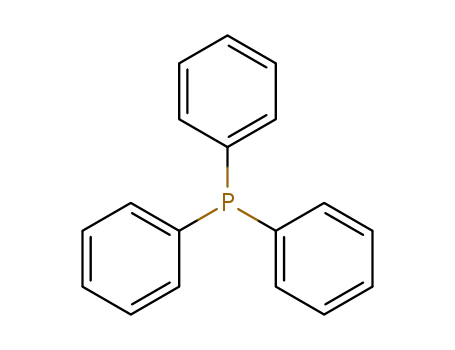
triphenylphosphine

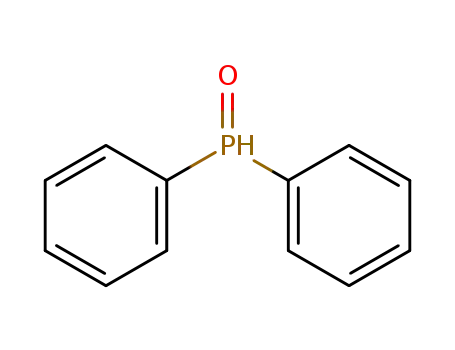
Diphenylphosphine oxide
| Conditions | Yield |
|---|---|
|
phenylmagnesium bromide; triethyl phosphite;
In
tetrahydrofuran;
Inert atmosphere;
Inert atmosphere;
Acidic aq. solution;
|
18% 5% 14% |
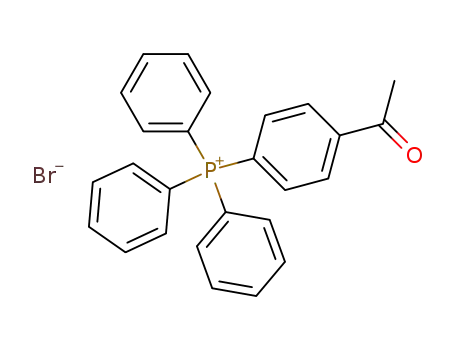
(p-acetylphenyl)triphenylphosphonium bromide

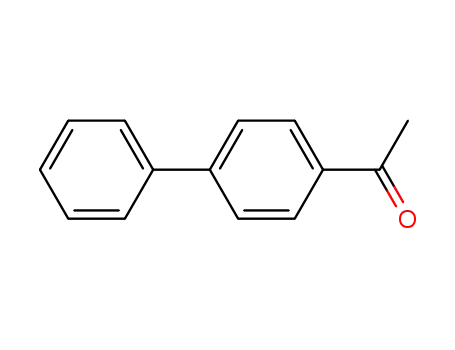
biphenyl-4-acetaldehyde

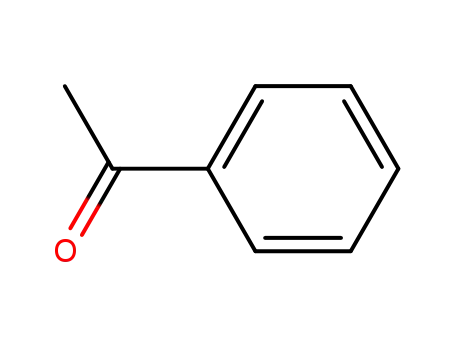
acetophenone


Diphenylphosphine oxide

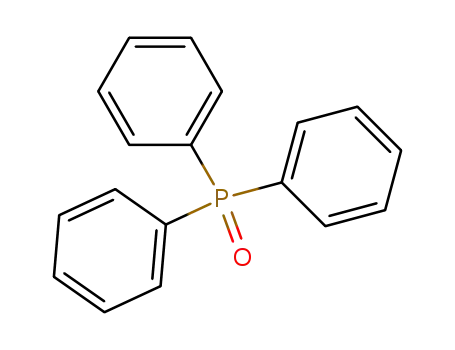
Triphenylphosphine oxide
| Conditions | Yield |
|---|---|
|
With
water; sodium hydroxide;
at 20 ℃;
|
47% 20% 22% 21% |
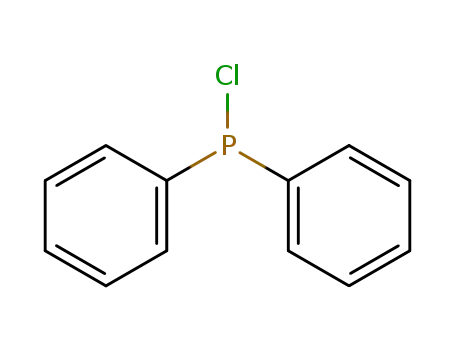
chloro-diphenylphosphine
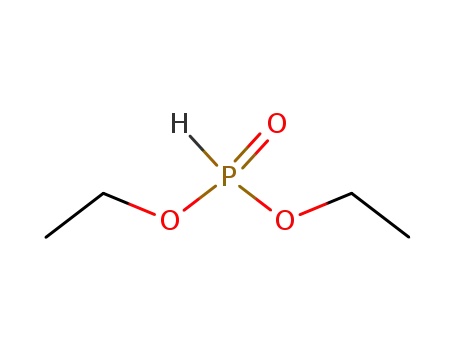
phosphonic acid diethyl ester
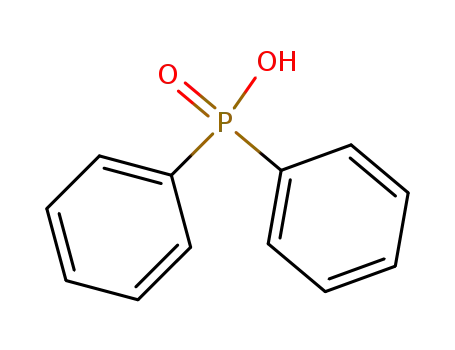
diphenyl-phosphinic acid
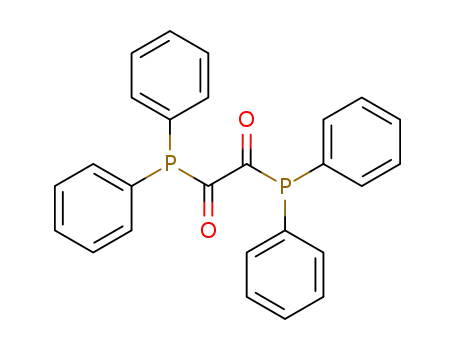
P,P'-tetraphenyl-oxalic acid diphosphide
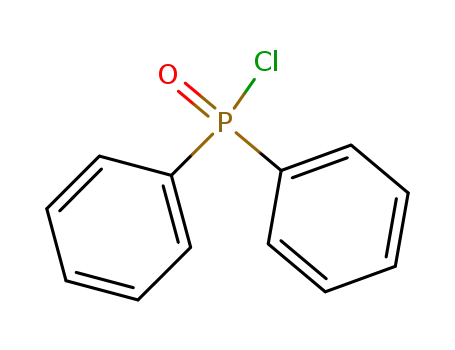
Diphenylphosphinic chloride

diphenyl-phosphinic acid
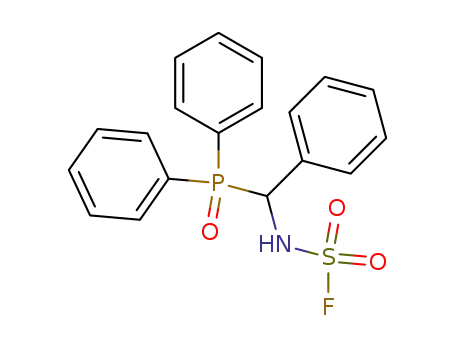
(α-Fluorsulfonylaminobenzyl)-diphenylphosphinoxid
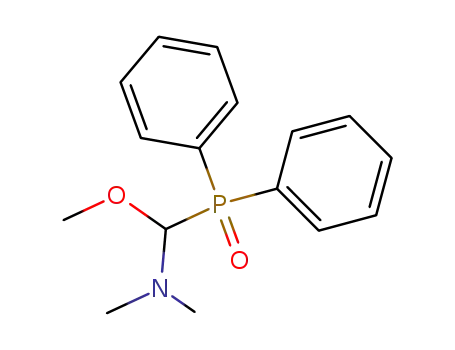
(dimethylamino)(diphenylphosphinoyl)methoxymethane
CAS:657408-07-6
Molecular Formula:C26H35O2P
Molecular Weight:410.5
CAS:1448787-63-0
CAS:161265-03-8
Molecular Formula:C39H32OP2
Molecular Weight:578.6