Your Location:Home >Products >Functional intermediates >952431-30-0

Product Details
The present invention relates to an organic electroluminescent compound applied to a hole transfer material, which is characterized by being presented by chemical formula 1-1 and chemical formula 1-2. The organic electroluminescent compound according to the present invention can manufacture an organic electroluminescent device having improved light emitting efficiency and life properties, when applied to a hole transfer layer of the organic electroluminescent device.
An amine-based compound and an organic light-emitting diode including the same are provided.
An organic EL device including an anode; an emission layer; an anode-side hole transport layer between the anode and the emission layer, the anode-side hole transport layer including an anode-side hole transport material and being doped with an electron accepting material; an intermediate hole transport layer between the anode-side hole transport layer and the emission layer, the intermediate hole transport layer including an intermediate hole transport material; and an emission layer-side hole transport layer between the intermediate hole transport layer and the emission layer, the emission layer-side hole transport layer being adjacent to the emission layer, wherein the emission layer-side hole transport layer includes an emission layer-side hole transport material represented by the following Formula 1:
An organic electroluminescent device of which emission life may be improved. The organic electroluminescent device includes an anode, an emission layer, and an anode-side hole transport layer provided between the anode and the emission layer and including an anode-side hole transport material. An electron accepting material is doped in the anode-side hole transport layer. An intermediate hole transport material layer is provided between the anode-side hole transport layer and the emission layer and includes an intermediate hole transport material, and an emission layer-side hole transport layer is provided between the intermediate hole transport material layer and the emission layer and adjacent to the emission layer. The emission layer-side hole transport layer includes an emission layer-side hole transport material represented by the following Formula 1.
![N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine](/upload/2023/2/64c329f9-47fa-4188-a888-413c06efde17.png)
N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine

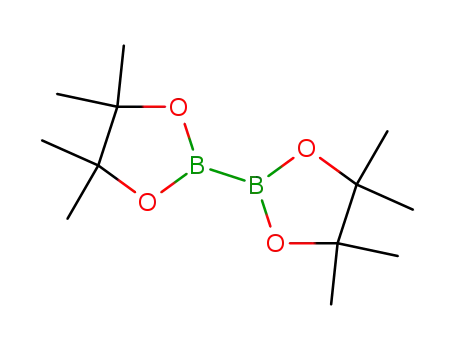
bis(pinacol)diborane

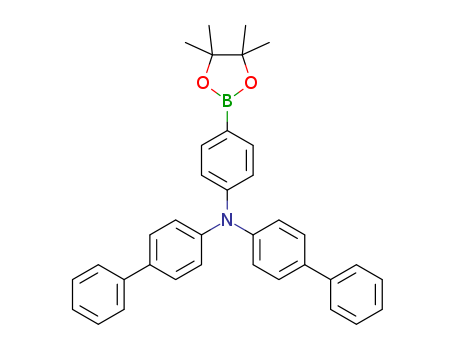
![N-[1,1-biphenyl]-4-yl-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-[1,1-biphenyl]-4-amine](/upload/2023/2/b83feda9-e461-4c91-bb48-13973b526eec.png)
N-[1,1-biphenyl]-4-yl-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-[1,1-biphenyl]-4-amine
| Conditions | Yield |
|---|---|
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
at 100 ℃;
for 12h;
Inert atmosphere;
|
98% |
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
at 100 ℃;
for 12h;
Inert atmosphere;
|
98% |
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
at 100 ℃;
for 12h;
Inert atmosphere;
|
98% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
toluene;
for 13h;
Reflux;
|
83% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; palladium diacetate;
In
toluene;
for 10h;
Reflux;
|
83% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
dimethyl sulfoxide;
at 80 ℃;
for 6h;
|
80% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
toluene;
for 12h;
Reflux;
|
77% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
toluene;
for 12h;
Reflux;
|
77% |
|
N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine; bis(pinacol)diborane;
With
potassium acetate;
In
1,4-dioxane;
for 0.25h;
Inert atmosphere;
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;
In
1,4-dioxane;
at 80 ℃;
for 18h;
Inert atmosphere;
|
75% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; palladium diacetate;
In
toluene;
for 12h;
Reflux;
|
71% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate; tert-butyl XPhos;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 60 - 120 ℃;
for 5h;
Inert atmosphere;
|
![N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine](/upload/2023/2/64c329f9-47fa-4188-a888-413c06efde17.png)
N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine

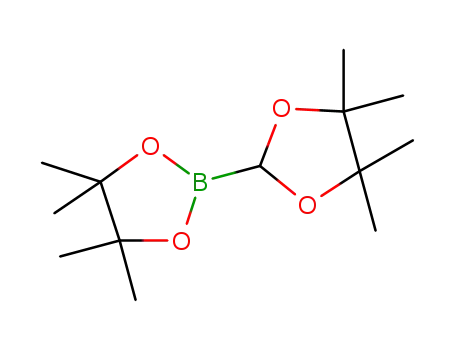
4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3-dioxolan-2-yl)-1,3,2-dioxaborolane

![N-[1,1-biphenyl]-4-yl-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-[1,1-biphenyl]-4-amine](/upload/2023/2/b83feda9-e461-4c91-bb48-13973b526eec.png)
N-[1,1-biphenyl]-4-yl-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-[1,1-biphenyl]-4-amine
| Conditions | Yield |
|---|---|
|
With
dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate;
In
1,4-dioxane;
Inert atmosphere;
Reflux;
|
71% |
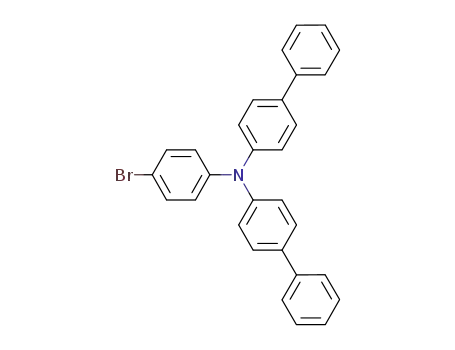
N-([1′,1′-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1′-biphenyl]-4-amine
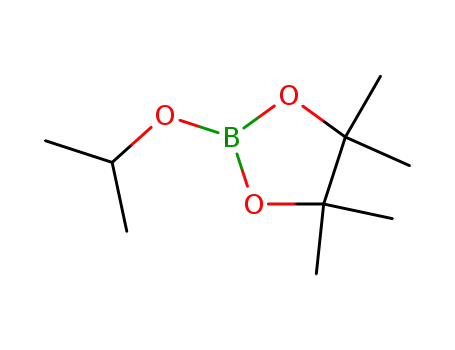
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

bis(pinacol)diborane
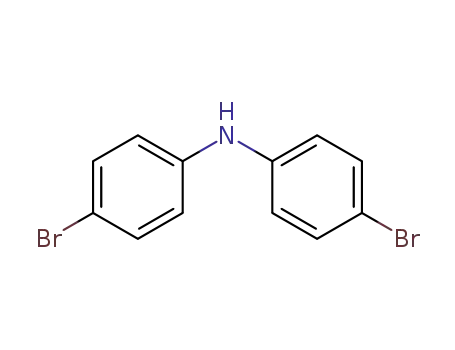
bis(4-bromophenyl)amine
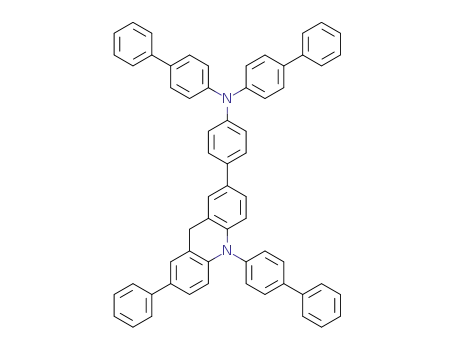
N-(biphenyl-4-yl)-N-(4-(10-(biphenyl-4-yl)-7-phenyl-9,10-dihydroacridin-2-yl)phenyl)biphenyl-4-amine
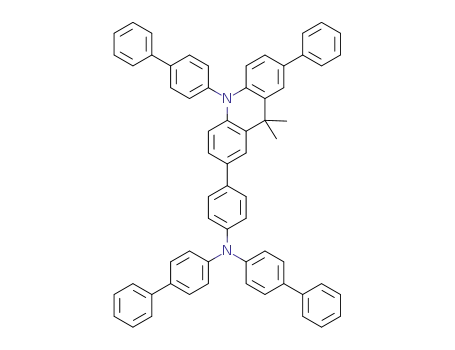
bis(biphenyl-4-yl)-[4-{10-(biphenyl-4-yl)-9,9-dimethyl-7-phenylacridan-2-yl}phenyl]amine
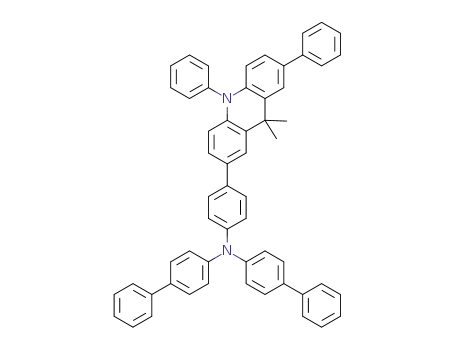
bis(biphenyl-4-yl)-[4-(9,9-dimethyl-7,10-diphenylacridan-2-yl)phenyl]amine
CAS:116971-11-0
CAS:577-19-5
CAS:1448787-63-0