Your Location:Home >Products >OLED intermediates >Thiophenes >104934-52-3
.jpg)
.jpg)
Product Details
Chemical Properties
clear colorless to yellow liquid
Uses
3-Dodecylthiophene is a reactant used to make semiconducting copolymers.
Uses
Conducting polymer precursor.
Preparation
3-Dodecylthiophene can be prepared from 3-bromothiophene and halogenated hydrocarbons in one step. Steps: Under N2 atmosphere, slowly add 1-bromododecane (28.75g, 26.9 mL) to a 250mL three-necked flask containing a mixture of magnesium chips (3.28g, 0.135mol), anhydrous THF (30mL) and a small amount of iodine. mL, 0.13 mol) in dry THF (45 mL). After the mixture was refluxed at 70 °C for 2 hours, the system was cooled to room temperature with ice water, Ni(dppp)Cl2 (0.54 g, 1.00 mmol) was added first, and then 3-bromothiophene (16.31 g, 0.10 mol) was added slowly. Anhydrous THF (40 mL) solution. The mixed solution was stirred at room temperature overnight, and cold aqueous HCl (1.50 mol/L) was added to quench the reaction. The crude product was extracted with dichloromethane, dried over anhydrous magnesium sulfate, and further purified by column separation purification (n-hexane as eluent), resulting in a clear liquid (22.18 g, yield=88%).
General Description
3-Dodecylthiophene (3-DT) is a conjugating monomer that can be used as an active layer on semiconductors. It has good electronic properties and can be used in the development of p-type semiconducting polymers. It is mainly used in the formation of poly(3-dodecylthiophene) (P3DT) through electrochemical polymerization. P3DT can further be utilized for a variety of organic electronic based applications.
InChI:InChI=1/C16H28S/c1-2-3-4-5-6-7-8-9-10-11-12-16-13-14-17-15-16/h13-15H,2-12H2,1H3
Regioselective bromination of alkylated bi- and ter-thiophenes affords key building blocks for the synthesis of higher, isomerically pure alkyl oligothiophenes.For all new compounds, unequivocal structural assignment is based on a detailed analysis of the fully coupled 13C, 1H NMR spectra.
In this study, we obtained a new structural insight into the charge-transporting properties in TPD-based polymers that cannot be solely explained in terms of the type of orientation. We synthesized two types of copolymers comprising mono-TPD or bis-TPD as the accepting unit. Although the planarity and energy levels are similar with the mono-TPD unit, the aggregation state is quite different, and the X-aggregation tendency seems to be stronger when the bis-TPD unit is incorporated. In the case of TPD1, an effective π-πorbital overlap is found to originate from the H-aggregates, and 3D charge transport pathways are formed with a bimodal orientation of edge-on and face-on, resulting in an efficient charge transportation (1.84 cm2·V-1·s-1 of hole and 0.31 cm2·V-1·s-1 of electron). In contrast, despite the well-aligned edge-on orientation of TPD2, it exhibited a relatively very low mobility and splitted emission characteristics in photoluminescence spectra because of the tilted intermolecular stacking pattern with an X-shape (0.015 cm2·V-1·s-1 for hole and 0.16 cm2·V-1·s-1 for electron). An overall characterization of the semiconducting polymers was performed, and it was found that the type of aggregation in the final thin films, such as H- or X-aggregation, is indeed important and perhaps more important than the orientation to obtain polymers with a high charge carrier mobility.
Two D-A copolymers, F1 and F2, with fluorene and thiazole units were substituted, respectively, on a thiadiazoloquinoxaline (TDQ) unit to enhance the electron-accepting strength of TDQ. The copolymers were synthesized by a cross-coupling Stille reaction and their optical and electrochemical properties were examined, which revealed that they have ultra-low band gaps and absorption in the near-infrared. These copolymers were employed as donors along with PC71BM as an electron acceptor for the fabrication of solution-processed bulk heterojunction (BHJ) polymer solar cells. After the optimization of the donor-to-acceptor weight ratio and the solvent additive (4 v% DIO as solvent additive), devices with F1:PC71BM and F2:PC71BM displayed power conversion efficiencies (PCEs) of 5.80% and 3.32%, respectively. Although F2 possesses a broader absorption profile compared with F1, the lower value of PCE for the F2-based device was attributed to the low LUMO offset between F2 and PC71BM, which limited the exciton dissociation. The abovementioned results indicate that these copolymers can be utilized for ternary BHJ and tandem solar cells to achieve a high PCE.
Precise control of the molecular arrangements at the interface between the electron donor and acceptor in mixed bulk heterojunctions (BHJs) remains challenging, despite the correlation between structural characteristics and efficiency in organic photovoltaics (OPVs). This study reveals that the substitution patterns of linear and branched alkyl side chains on electron-donating/-accepting alternating copolymers can control the positions of an acceptor molecule (C60) around the π-conjugated main chains in mixed BHJs. Two-dimensional solid-state NMR demonstrates a marked difference in the location of C60 in the blend films. A copolymer with an electron-accepting unit positioned in close proximity to C60 demonstrated higher OPV performance in combination with various fullerene derivatives. This molecular design offers precise control over the interfacial molecular structure, thereby paving the way for overcoming the current limitations of OPVs comprising mixed BHJs.
New polymeric azines, DOZ and DOPh, containing the 3-dodecylthiophene moiety, have been obtained by the condensation reactions of 2,5-diformyl-3-dodecylthiophene with hydrazine and p-diaminobenzene, respectively. Their average molecular masses depend on t
The synthesis of poly(thienylene vinylenes) (PTV) has been attracting attention due to the low band gaps and high electrical conductivities of these materials, making them applicable for charge storage devices, transparent conductive coatings, and electrochromic devices. Unsubstituted PTV is an intractable polymer that is usually synthesized via a processable precursor. This article reports the synthesis of conjugated polymers using solid-state metathesis conditions, demonstrating the efficiency of this methodology for preparation of a processable polymer, such a 3-dodecyl PTV (P3DDTV). The 3-dodecyl-2,5-dipropenylthiophene was synthesized and subsequently polymerized using ADMET conditions with Grubbs' second-generation catalyst. The prepolymer film (Mn = 4000 g/mol) was further polymerized in the solid state to give a final product with Mn = 14 000 g/mol (a 3.5-fold increase while in the solid state). The polymer obtained by this methodology exhibited thermal (Tg = 43 °C and Tm = 115 °C) and electrochemical (optical band gap of 1.65 eV and HOMO energy level of 5.35 eV) properties similar to those of PTV polymers synthesized by ADMET polymerization using a high boiling solvent or by cross-coupling reactions.
Acceptor-donor-acceptor-type compounds 5′,5″-(benzo[c][1,2,5]selenadiazole-4,7-diyl)bis(3′-dodecyl-2,2′-bithiophene-5-carbonitrile) (9) and 4,4′-(5,5′-(benzo[c][1,2,5]selenadiazole-4,7-diyl)bis(3-dodecylthiophene-5,2-diyl))dibenzonitrile (10) were designed and synthesized. These compounds differ in terminal positions, compound 9 with a thiophene-containing nitrile group and compound 10 with a phenyl-containing nitrile group. Both compounds have shown good thermal stability and low band gap. The band gaps of compounds 9 and 10 were 1.74 and 1.83 eV, respectively. These results indicate that they are promising materials for use in optoelectronics.
The synthesis of a poly(1,3-dithienylisothianaphthene) (PDTITN) derivative obtained via oxidative polymerization of 5,6-dichloro-l,2-bis(3a?2-dodecylthienyl)isothianaphthene is described. PDTITN exhibits a band gap of 1.80-1.85 eV. The redox properties of PDTITN were characterized using cyclic voltammetry and spectroelectrochemisty. Photoexcitation of PDTITN results in photoluminescence (PL) in the near-IR region and the formation of a triplet state. In the presence of a methanofullerene (PCBM) as an electron acceptor, PL and triplet formation of PDTITN are quenched. In photovoltaic devices using blends of the polymer with PCBM, the observed incident-photon-to-collected-electron efficiency (IPCE) up to 24% at 400 nm and the 3 orders of magnitude increase of short circuit current as compared to the polymer alone prove the photoactivity of the PDTITN/PCBM blend in the device.
A series of conjugated polymers containing thienylenevinylene moieties as the electron donor for polymer solar cells were synthesized through Yamamoto and Stille coupling. The structure and device properties of the homopolymer (E)-poly[2,2′-(1,2-ethenediyl)bisthiophene] (PEBT), and two donor-acceptor-type copolymers (E)-poly[2,2′-(1,2-ethenediyl)bisthiophene- alt-4,7-(2,1,3-benzothiadiazole)] (PEBTBT) and (E)-poly[2,2′-(1,2- ethenediyl)bisthiophene-alt-5,5-(4′,7′-di-2-thienyl-2′, 1′,3′-benzothiadiazole)] (PEBTTBT) were investigated. The vinylene group inserted between two thiophene rings has an important role in lowering the band gap of thienylenevinylene derivatives. In addition, the donor-acceptor-type copolymers including a benzothiadiazole unit showed even lower band gap than their own homopolymer due to intramolecular charge transfer (ICT) through their conjugated backbones. As a result, PEBT, PEBTBT and PEBTTBT showed band gaps of 1.77, 1.37 and 1.57 eV, respectively. Bulk heterojunction photovoltaic devices were fabricated using blends of the polymers with [6,6]-phenyl C71-butyric acid methyl ester (PC70BM) and PEBTBT gave the best power conversion efficiency among them, at 1.07% under AM 1.5, 100 mW cm-2. The Royal Society of Chemistry 2011.
Donor-acceptor-based poly(E)-4-(3,4′-didodecyl-5′-(2-(3-dodecylthiophen-2-yl)vinyl)-2,2′-bithiophen-5-yl)-7-(4-dodecylthiophen-2-yl)benzo[c][1,2,5]selenadiazole (11) has been synthesized by a Stille coupling reaction. This polymer has a low energy band gap between the HOMO and LUMO energy levels of 1.75 eV. The polymer exhibited good thermal stability. An OTFT prepared using this polymer displayed high hole mobility of 0.097 cm2 V-1 s-1 at 200°C, a high on/off ratio of 7.8 × 104, and a low threshold voltage of 11.2 V. When compared with as-cast films, annealed films exhibited higher mobility, which was attributed to an increase in crystallinity with an increase in the annealing temperature.
Bolaamphiphilies with D-A-D type π-conjugated rigid cores composed by thiophenes as donors (D) and benzothiadiazole (BTD) as central acceptor (A) have been synthesised. Their self-assemblies and photophysical properties were investigated by polarising optical microscope, differential scanning calorimetry, X-ray diffraction and scanning electron microscopy. Such compounds can self-assemble into honeycomb cylinder mesophases with Colhex?/p6mm and Colsqu/p4mm lattices in their pure states as well as organogels with different morphologies in organic solvents. Their absorption spectra cover nearly the entire visible light range and their band gaps are relatively low. Tetrathiophene BTD based bolaamphilphiles (BT4/n) with higher D/A ratios than the bisthiophene BTD bolaamphilphiles (BT2/n) can self-assemble into more ordered nanostructures in both bulk states and solution. Both the absorption and emission peaks of BT4/n are strongly red shifted. The influence of the molecular conformation, the conjugated core length, as well as the D/A ratio on the self-assemble and photophysical characteristics of such D-A-D bolaamphiphiles are discussed.
Provided is a method of synthesizing a C(sp3)-C(sp2) cross-coupled compound comprising reacting a C(sp3) coupling partner with a C(sp2) coupling partner, a catalyst, and a solvent; wherein the C(sp3) coupling partner comprises an organic zinc reagent; and wherein the C(sp2) coupling partner comprises a heterocyclic halide or a heterocyclic pseudo halide. The method further comprises synthesis of the organic zinc reagent, wherein the synthesis comprises reacting a zinc powder with an acid, filtering, washing, and drying to obtain an activated zinc powder; and reacting the activated zinc powder with a metal iodide catalyst and a second solvent and heating for a predetermined time to obtain the organic zinc reagent.
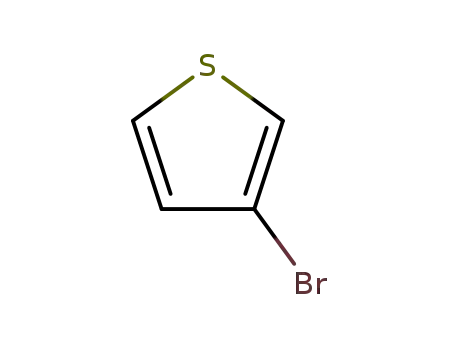
3-Bromothiophene


1-dodecylbromide


3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
1-dodecylbromide;
With
iodine; magnesium;
In
tetrahydrofuran;
at 70 ℃;
for 2h;
Inert atmosphere;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
at 20 ℃;
|
88% |
|
1-dodecylbromide;
With
iodine; magnesium;
In
diethyl ether;
for 2h;
Heating;
3-Bromothiophene;
With
nickel dichloride;
In
diethyl ether;
at 20 ℃;
Further stages.;
|
77% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
diethyl ether;
|
70% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
diethyl ether;
|
70% |
|
1-dodecylbromide;
With
iodine; magnesium;
In
tetrahydrofuran;
at 70 ℃;
for 5h;
Inert atmosphere;
Schlenk technique;
3-Bromothiophene;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
Inert atmosphere;
Schlenk technique;
Reflux;
|
60% |
|
With
pyridine; manganese; Tri(p-tolyl)phosphine; cobalt(II) bromide;
In
N,N-dimethyl acetamide;
at 70 ℃;
for 24h;
|
48% |
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) Et2O, reflux, 2 h, 2.) Et2O, reflux, 20 h;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
Yield given. Multistep reaction;
1.) Et2O, 0 deg C, overnight, 2.) Et2O, reflux, 3 h;
|
|
|
With
magnesium;
In
diethyl ether;
|
|
|
3-Bromothiophene; 1-dodecylbromide;
With
magnesium;
In
diethyl ether;
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
tetrahydrofuran;
|
|
|
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium;
In
tetrahydrofuran;
|

3-Bromothiophene

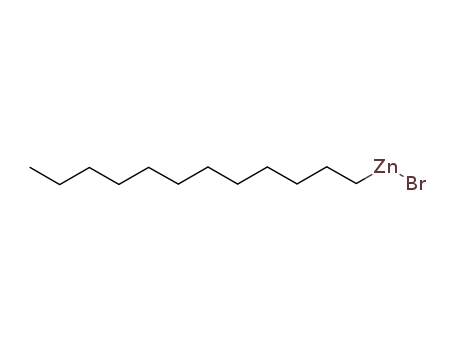
dodecylzinc(II) bromide


3-dodecylthiophene
| Conditions | Yield |
|---|---|
|
With
[1,1'-bis(diphenylphosphino)-ferrocene]palladium(II) chloride-dichlormethane complex;
In
N,N-dimethyl acetamide;
at 80 ℃;
for 12h;
|
95% |

3-Bromothiophene
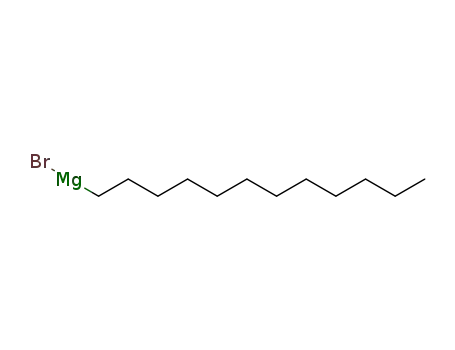
laurylmagnesium bromide

1-dodecylbromide
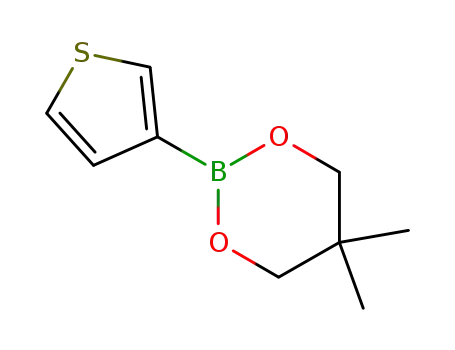
5,5-dimethyl-2-(thiophen-3-yl)-1,3,2-dioxaborinane
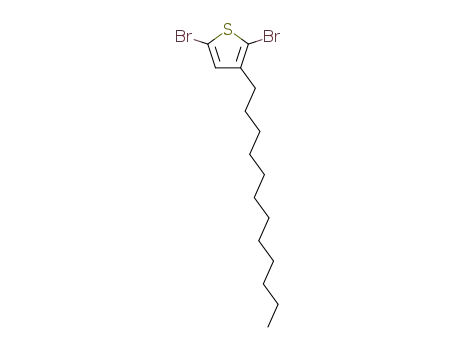
3-n-dodecyl-2,5-dibromothiophene

2-bromo-3-dodecylthiophene
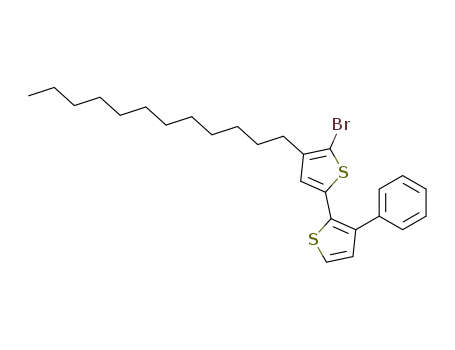
5'-bromo-4'-dodecyl-3-phenyl-[2,2']-bithiophene
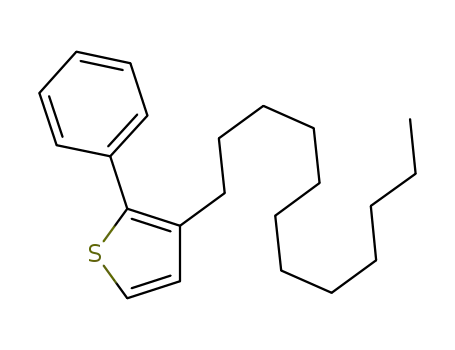
3-dodecyl-2-phenylthiophene
CAS:247940-06-3
CAS:19999-87-2
CAS:139100-06-4
CAS:110851-66-6