Your Location:Home >Products >OLED intermediates >Thiophenes >116971-11-0


Product Details
Chemical Properties
Colorless to yellow liquid
Uses
Conducting polymer precursor.
General Description
2,5-Dibromo-3-hexylthiophene is a 2,5 coupled conductive polymer with conjugated polythiophene based system, which has a controllable band gap.
InChI:InChI=1/C10H14Br2S/c1-2-3-4-5-6-8-7-9(11)13-10(8)12/h7H,2-6H2,1H3
We report on the solution-state assembly of all-conjugated polythiophene diblock copolymers containing nonpolar (hexyl) and polar (triethylene glycol) side chains. The polar substituents provide a large contrast in solubility, enabling formation of stably suspended crystalline fibrils even under very poor solvent conditions for the poly(3-hexylthiophene) block. For appropriate block ratios, complexation of the triethylene glycol side chains with added potassium ions drives the formation of helical nanowires that further bundle into superhelical structures.
Bromination in concentrated solutions of substituted thiophenes in acetic acid with NBS at room temperature was studied. Under the conditions, bromination readily took place with high regioselectivity at the 2-ring positions, > 99%. The developed method was demonstrated to be suitable for multimolar preparations.
Two polythiophene derivatives using fluorine atoms and hexyl or hexyloxy group as electron-withdrawing and donating substituents have been synthesized. The introduction of fluorine atoms to the polythiophene backbones simultaneously lowers the HOMO and narrows the bandgap, and the stronger electron-donating ability of hexyloxy side chain further reduces the bandgap. As a result, poly[3-hexylthiophene-2,5-diyl-alt-3,4-difluorothiophene] (PHTDFT) shows HOMO and bandgap of -5.31/1.83 eV and poly[3,4-dihexyloxythiophene-2,5-diyl-alt-3,4- difluorothiophene] (PDHOTDFT) shows HOMO and bandgap of -5.14/1.68 eV, both are lower than -4.76/2.02 eV of P3HT. Benefiting from the lower HOMO, PHTDFT:PC 61BM (1:1) polymer solar cells obtain a power conversion efficiency of 1.11% and an impressed open-circuit voltage of 0.79 V under solar illumination AM1.5 (100 mW/cm2). Two new polythiophene derivatives incorporating with 3,4-difluorothiphene units, PHTDFT and PDHOTDFT, show lower HOMO and narrower bandgap than that of P3HT. Benefiting from the lower HOMO, PHTDFT:PC61BM (1:1) polymer solar cells obtain a power conversion efficiency of 1.11% and an impressed open-circuit voltage of 0.79 V under solar illumination AM1.5 (100 mW/cm2). Copyright
Organometallic wire-like complexes with ferrocenyl termini, conjugated with one and two units of thienylethynyl (M1 and M2) and thienyl (M3 and M4) groups, have been synthesized with a general formula of [Fc-C≡C-(Th-C≡C)1-2-Fc] and [Fc-(Th)1-2-Fc] (Fc = ferrocenyl, Th = thienyl) respectively. The diferrocenyl organometallic complexes have been characterized by various spectroscopic tools such as multinuclear NMR, FTIR, elemental analysis, and mass spectrometry. The electrochemical properties of these compounds have been investigated by cyclic (CV) and differential pulse voltammetry (DPV). The single reversible oxidation wave in diferrocenyl complexes with thienylethynyl spacers (M1 and M2) indicates the absence of intramolecular electrochemical communication between the two ferrocenyl termini. Interestingly, the diferrocenyl complexes with one and two thienyl spacers (M3 and M4) show two successive reversible one-electron oxidation waves, indicating electronic coupling between the two ferrocenyl (Fc) termini. The potential difference (ΔE1/2) between the two redox centers is 160 mV and 130 mV, respectively, with the corresponding comproportionation constants (Kc) of 6.2 × 102 and 1.5 × 102. Upon mono-oxidation of M3 by [Cp2Fe][BF4], a broad and weak intervalence charge-transfer (IVCT) transition is observed in the NIR region of 1200–2200 nm.
We report a facile macroligand strategy towards the synthesis of low-bandgap inorganic-organic composites comprised of semiconductor PbS nanoparticles and functional copolymers. For this, thiol-functional thiophene-based macroligands have been used as coligands for PbS nanoparticles. Thus, solution processable organic-inorganic hybrid materials with absorption in the near-infrared have been obtained. The resulting nanoparticle-polymer composites were characterized in detail by optical and FT-IR spectroscopy as well as TEM showing their potential as novel functional inorganic-organic hybrid materials when applied in initial proof-of-concept hybrid photovoltaic devices.
We report the synthesis, morphology, and charge-transporting characteristics of new crystalline-crystalline diblock copolymers, poly(3-hexylthiophene-block-stearyl acrylate) (P3HT-b-PSA). Three different diblock copolymers, P1, P2, and P3, with P3HT/PSA polymerization degree block ratios of 60/26, 60/50, and 60/360, respectively, were prepared for investigating the morphology-property relationship and the dependence of optoelectronic properties on the block copolymer structure. Small- and wide-angle X-ray scattering indicated the presence of both P3HT and PSA crystalline domains and the presence of microphase separation among blocks. The transmission electron microscopy and atomic force microscopy results revealed that the diblock copolymers cast from chlorobenzene, tended to form needle-like morphologies. The field-effect mobilities of the diblock copolymers deposited on untreated SiO2 substrates, decreased with increasing PSA block length. In a sharp contrast, the mobilities enhanced with increasing PSA content when the P3HT-b-PSA was deposited on phenyltrichlorosilane (PTS)-treated substrates. The copolymers with a 60/360 P3HT/PSA ratio showed a good mobility of 4 × 10-3 cm2 V-1 s-1 and a high on/off ratio of 7 × 106 on PTS-treated substrates. This study highlighted the importance of the block ratio, the substrate and self-assembly structures on the charge transport characteristics of the crystalline-crystalline conjugated diblock copolymers.
Selenophene-thiophene block copolymers were synthesized and studied. The properties of these novel block copolymers are distinct from those of statistical copolymers prepared from the same monomers with a similar composition. Specifically, the block copolymers exhibit broad and red-shifted absorbance features and phase-separated domains in the solid state. Scanning transmission electron microscopy and topographic elemental mapping confirmed that the domains are either rich in selenophene or thiophene, indicating that the blocks of distinct heterocycles preferentially associate with one another in the solid state. This preference is surprising in view of the chemical similarities between repeat units. The overall results demonstrate a phase separation that is controlled by elemental differences. As a result of this phase separation, these novel conjugated block copolymers should find utility in a variety of studies and optoelectronics uses.
Organometallic molecular wires with π-conjugation along their molecular backbones are of considerable interest for application in molecular-scale electronics. In this regard, thienylethynyl-based π-conjugated oligomers of three, five and seven thienylethynyl units with -CC-H termini have been successfully synthesized through stepwise Pd(0)/Cu(i)-catalyzed Sonogashira coupling. The corresponding highly soluble diruthenium(ii) diacetylide complexes (O1-Ru2, O3-Ru2, O5-Ru2 and O7-Ru2, respectively) have been prepared by the reaction of cis-Ru(dppe)2Cl2 and NaPF6 in DCM with the corresponding rigid rod-like thienylethynyl oligomers with one, three, five and seven thienylethynyl π-conjugated segments containing alkynyl termini (O1, O3, O5 and O7). These Ru(ii)-Cl capped diacetylide complexes have been further functionalized by incorporating a phenylacetynyl moiety to afford [Ru(ii)-CC-Ph]-capped diacetylide organometallic wires (O1-Ru2-Ph, O3-Ru2-Ph, O5-Ru2-Ph and O7-Ru2-Ph). The photophysical properties of the highly soluble thienylethynyl-based oligomers and Ru(ii)-organometallic wires have been explored to understand their electronic properties. Electrochemical studies of the binuclear ruthenium(ii)-alkynyl complexes showed highly interesting results, revealing long-range electrochemical communication between the two remote Ru(ii) termini connected even with five and seven thienylethynyl units. DFT computational studies further support the long range electrochemical communication between the redox active metal termini through heavy participation of the thienylethynyl bridge in the corresponding mono-oxidized mixed valence species of the organometallic wire-like complexes.
We first report on the copolymerization of sulfur and allyl-terminated poly(3-hexylthiophene-2,5-diyl) (P3HT) derived by Grignard metathesis polymerization. This copolymerization is enabled by the conversion of sulfur radicals formed by thermolytic cleavage of S8 rings with allyl end-group. The formation of a C-S bond in the copolymer is characterized by a variety of methods, including NMR spectroscopy, size exclusion chromatography, and near-edge X-ray absorption fine spectroscopy. The S-P3HT copolymer is applied as an additive to sulfur as cathode material in lithium-sulfur batteries and compared to the use of a simple mixture of sulfur and P3HT, in which sulfur and P3HT were not covalently linked. While P3HT is incompatible with elementary sulfur, the new S-P3HT copolymer can be well dispersed in sulfur, at least on the sub-micrometer level. Sulfur batteries containing the S-P3HT copolymer exhibit an enhanced battery performance with respect to the cycling performance at 0.5C (799 mAh g-1 after 100 cycles for S-P3HT copolymer versus only 544 mAh g-1 for the simple mixture) and the C-rate performance. This is attributed to the attractive interaction between polysulfides and P3HT hindering the dissolution of polysulfides and the charge transfer (proven by electrochemical impedance spectroscopy) due to the homogeneous incorporation of P3HT into sulfur by covalently linking sulfur and P3HT.
Rieke zinc has a wide potential for applications in organic chemistry, notably for the synthesis of highly chemoselective organozinc reagents. However, due to the rather unreliable preparation method, leading to large batch to batch variations, its use has been rather limited. Rieke zinc is commonly prepared by the reduction of zinc chloride with lithium using a stoichiometric amount of naphthalene. In our hands, it was observed that the reaction outcome was highly dependent on the naphthalene source and purity grade. The presence of benzothiophene seems crucial to avoid coagulation of the zinc particles and the amount of benzothiophene has a large effect on the physical properties and the reactivity of the resulting zinc powder. Accordingly, highly reactive Rieke zinc was easily prepared from zinc chloride by adding an optimum amount (3 mol% with regard to ZnCl2) of benzothiophene into the lithium naphthalenide solution (prepared in situ). The Rieke zinc obtained in this way was successfully employed in the so-called "Rieke method" to prepare regioregular poly(3-hexylthiophene) (P3HT). The novel approach enables preparation of Rieke zinc in a reproducible manner, which considerably facilitates its use in both small molecule and polymer synthesis.
A series of poly(3-hexyl thiophene) (P3HT)-based diblock copolymers were prepared and examined in solution for their assembly into fibrils, and post-assembly cross-linking into robust nanowire structures. P3HT-b-poly(3-methanol thiophene) (P3MT), and P3HT-b-poly(3-aminopropyloxymethyl thiophene) (P3AmT) diblock copolymers were synthesized using Grignard metathesis (GRIM) polymerization. Fibrils formed from solution assembly of these copolymers are thus decorated with hydroxyl and amine functionality, and cross-linking is achieved by reaction of diisocyanates with the hydroxyl and amine groups. A variety of cross-linked structures, characterized by transmission electron microscopy (TEM), were produced by this method, including dense fibrillar sheets, fibril bundles, or predominately individual fibrils, depending on the chosen reaction conditions. In solution, the cross-linked fibrils maintained their characteristic vibronic structure in solvents that would normally disrupt (dissolve) the structures.
A library of symmetrical linear oligothiophene was prepared employing decarboxylative cross-coupling reaction as the key transformation. Thiophene potassium carboxylate salts were used as cross-coupling partners without the need of co-catalyst, base, or additives. This method demonstrates complete chemoselectivity and is a comprehensive greener approach compared to the existing methods. The modularity of this approach is demonstrated with the preparation of discreet oligothiophenes with up to 10 thiophene repeat units. Symmetrical oligothiophenes are prototypical organic semiconductors where their molecular electrical doping as a function of the chain length can be assessed spectroscopically. An oligothiophene critical length for integer charge transfer was observed to be 10 thiophene units, highlighting the potential use of discrete oligothiophenes as doped conduction or injection layers in organic electronics applications.
A series of highly emissive π-conjugated A-alt-B type copolymers (P1-P3) appended with a 1,2,3-triazole moiety was synthesized via Suzuki polymerization. The well-defined and soluble π-conjugated copolymers were characterized via multinuclear NMR spectroscopy and tetradetector GPC studies, showing a molecular weight (Mn) in the range of 16.4-20.1 kDa with a polydispersity index in the range of 1.25-1.42. The synthesized emissive π-conjugated polymer probes were explored as fluorescent chemosensors for nitroaromatic compounds (NACs) in solution, vapor and contact mode. Detailed photophysical and sensing studies were performed to understand the polymer-NAC interaction, inducing the selective fluorescence quenching of the π-conjugated polymer probes through the photoinduced electron transfer (PET) mechanism. All the polymeric probes (P1-P3) were highly reversible in nature with NACs, and thus could be reused multiple times. The limit of detection of the probes towards nitroaromatics was found to be in the range of 120-200 ppb with a high association constant in the order of 104 M-1. Furthermore, test paper kits were also fabricated, which allowed the trace detection of picric acid by the naked eye, making it a practical means for the quick, easy and inexpensive on-site detection of NAC-based explosives.

3-hexylthiophene

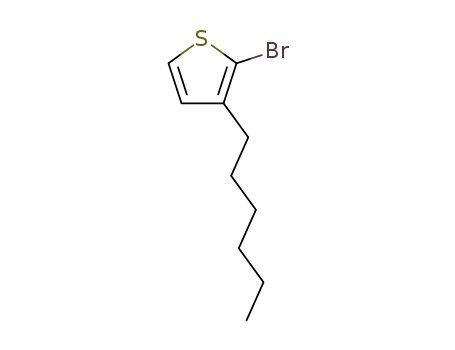
3-hexyl-2-bromothiophene

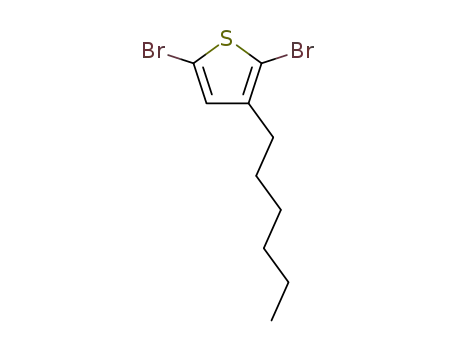
2,5-dibromo-3-hexylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.833333h;
Yields of byproduct given;
|
91.7% |

3-hexylthiophene


2,5-dibromo-3-hexylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
chloroform;
at 0 ℃;
|
99% |
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 60 ℃;
|
98% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
water;
at -5 - 20 ℃;
for 23h;
Product distribution / selectivity;
|
97% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 0.5h;
Heating;
|
95% |
|
With
methanol; tetrabutylammomium bromide; bromine;
In
dichloromethane;
at 20 ℃;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 12h;
Inert atmosphere;
Schlenk technique;
|
92% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 4.16667h;
Inert atmosphere;
|
88% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 12h;
|
88% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
81% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
81% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
Inert atmosphere;
|
80% |
|
With
N-Bromosuccinimide;
at 20 ℃;
Cooling with ice;
|
80% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 22.5 ℃;
for 16h;
Inert atmosphere;
|
80% |
|
With
N-Bromosuccinimide;
In
chloroform; N,N-dimethyl-formamide;
at 60 ℃;
Inert atmosphere;
|
71% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 5h;
|
70% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
69% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
Darkness;
|
64% |
|
With
bromine;
In
chloroform;
Ambient temperature;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
Inert atmosphere;
|
8.5 g |
|
3-hexylthiophene;
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 1h;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran; chloroform;
at 20 ℃;
Cooling with ice;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 28 ℃;
for 12h;
|

3-hexylthiophene

3-hexyl-2-bromothiophene
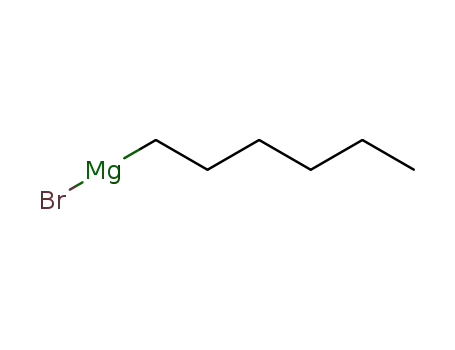
n-hexylmagnesium bromide
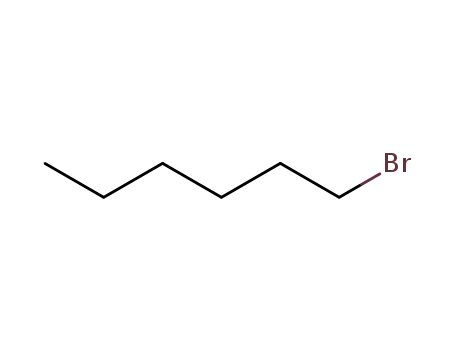
1-bromo-hexane
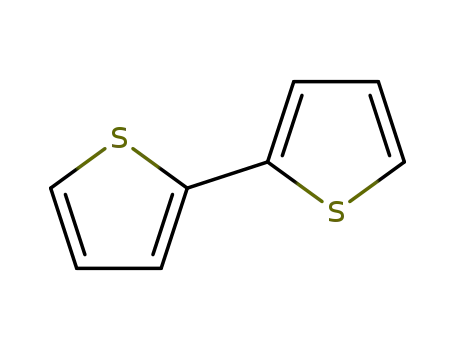
2,2'-Bithiophene
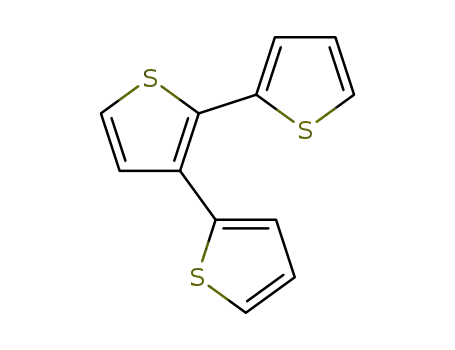
2,2':3',2-terthiophene
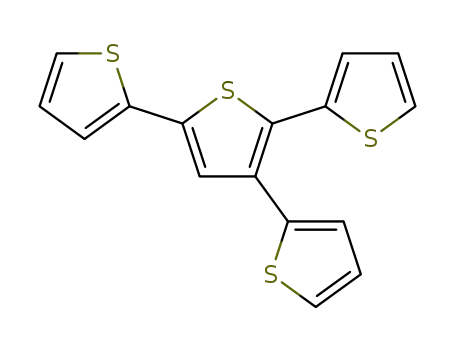
5'-(2-thienyl)-2,2':3',2-terthiophene
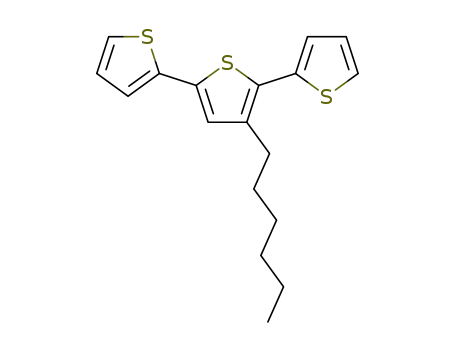
3'-hexyl-2,2':5',2-terthiophene
CAS:108756-88-3
CAS:1448787-63-0
CAS:825-55-8
CAS:69249-61-2