Your Location:Home >Products >OLED intermediates >Fluorenes >186259-63-2
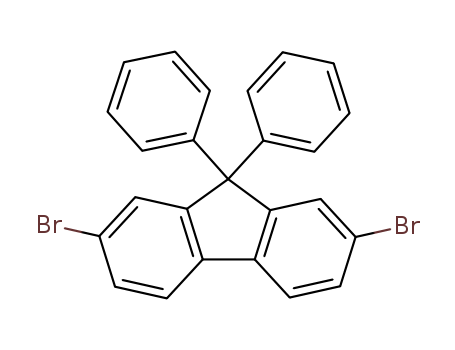

Product Details
InChI:InChI=1/C25H16Br2/c26-19-11-13-21-22-14-12-20(27)16-24(22)25(23(21)15-19,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-16H
Two series of Pt(II) polyynes bearing fluorene-type ligands with ethynyl units at different positions have been synthesized. In the absorption spectra, the Pt(II) polyynes bearing fluorene-type ligands with ethynyl units at 3,6-position have blue-shift with respect to the corresponding analogs bearing fluorene-type ligands with ethynyl units at 2,7-position, showing better transparency in the visible light region. Moreover, the Pt(II) polyynes bearing fluorene-type ligands with ethynyl units at 3,6-position show stronger triplet emission than corresponding analogs bearing fluorene-type ligands with ethynyl units at 2,7-position in the photoluminescent (PL) spectra. Furthermore, these Pt(II) polyynes were applied to optical power limiting (OPL) field. The Pt(II) polyynes bearing fluorene-type ligands with ethynyl units at 2,7-position show better OPL performance than the corresponding analogs with fluorene-type ligands of ethynyl units at 3,6-position. Therefore, changing the position of the ethynyl units in fluorene-type ligands can not only effectively control the photophysical properties of the Pt(II) polyynes, but also has an important effect on their OPL ability.
The invention provides a benzfluorene derivative and an organic light-emitting device containing the same, and relates to the technical field of organic optoelectronic materials. According to the benzfluorene derivative and the organic light-emitting device containing the same, by connecting a substituted or unsubstituted aromatic-ring condensed imidazole derivative and aromatic-ring condensed oxazole derivative onto a benzfluorene main body, the benzfluorene derivative is obtained. The benzfluorene derivative has certain electron transportation capability, is beneficial to compounding of holes and electrons in a light-emitting layer and has good stability and high light-emitting efficiency, synthesis is simple and easy to operate, and the benzfluorene derivative can be applied to the organic light-emitting device as a light-emitting layer doped material, so that the problems can be effectively solved that in the organic light-emitting device, a blue light-emitting material is low in light-emitting efficiency and short in service life, and the organic light-emitting device containing the same has the advantages of being high in light-emitting efficiency and long in service life.
The invention provides a fluorene derivative and an organic light-emitting device prepared by the same and relates to the technical field of organic photoelectric materials. The fluorene derivative and the organic light-emitting device prepared by the same have the advantages that the fluorene derivative is prepared by connecting substituted or unsubstituted condensed imidazole and condensed oxazole derivatives to a fluorene host, the prepared fluorene derivative has certain electron transport ability, the composition of holes and electrons on a light-emitting layer is benefited, and the fluorene derivative is good in stability and luminous efficiency, simple to synthesize, easy in synthesizing operation, capable of being used in the organic light-emitting device to serve as the light-emitting layer doping material and capable of effectively solving the problems that the blue light-emitting material in the organic light-emitting device is low in luminous efficiency and short in servicelife; the organic light-emitting device is high in luminous efficiency and long in service life.
π-Expanded butterfly-like 2D fluorenes and 3D spirobifluorenes 1–5 were synthesized via a DDQ-mediated oxidative cyclization strategy with a high regioselectivity. Through structural modification via π-expansion, it was possible to achieve near-ultraviolet absorption, bright-blue emission, very high near-unity fluorescence quantum yields in solution as well as in film states, and deep-lying HOMO energy levels with excellent thermal stabilities. Furthermore, these electron-rich compounds displayed a notable behavior towards sensing of nitroaromatic explosives, such as picric acid, up to a detection limit of 0.2 ppb.
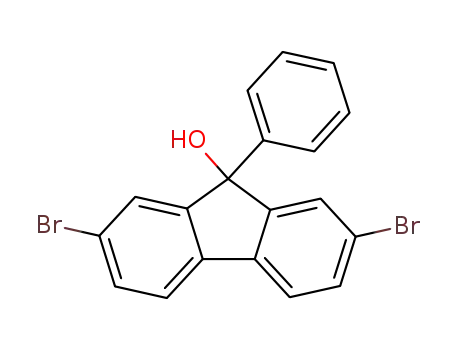
2,7-dibromo-(9-phenyl-9H-fluoren-9-ol)

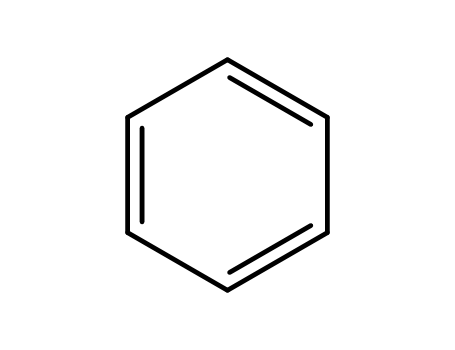
benzene

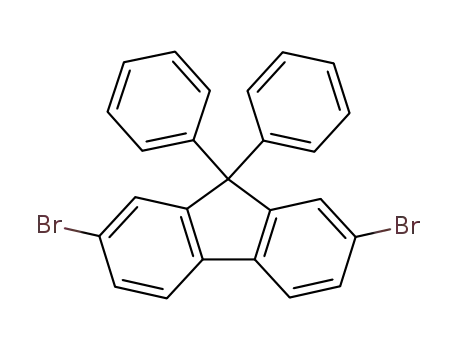
2,7-dibromo-9,9-diphenyl-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
trifluorormethanesulfonic acid;
at 80 ℃;
for 6h;
Reflux;
|
93% |
|
With
trifluorormethanesulfonic acid;
at 80 ℃;
for 6h;
|
88% |
|
With
trifluorormethanesulfonic acid;
at 50 - 80 ℃;
for 6h;
Inert atmosphere;
Schlenk technique;
|
70% |
|
With
sulfuric acid;
for 16h;
Inert atmosphere;
Reflux;
|
43% |
|
With
sulfuric acid;
for 3h;
|
|
|
2,7-dibromo-(9-phenyl-9H-fluoren-9-ol); benzene;
With
trifluorormethanesulfonic acid;
at 80 ℃;
for 6h;
Heating / reflux;
With
sodium hydrogencarbonate;
In
water;
at 0 ℃;
|
|
|
With
trifluorormethanesulfonic acid;
Heating;
|
|
|
With
trifluorormethanesulfonic acid;
|
9,9-diphenylfluorene


2,7-dibromo-9,9-diphenyl-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
bromine; acetic acid;
In
chloroform;
for 1h;
Inert atmosphere;
Reflux;
|
90% |

2,7-dibromo-(9-phenyl-9H-fluoren-9-ol)

benzene
2,7-dibromofluorene-9-one
phenyllithium
C37H40B2O4
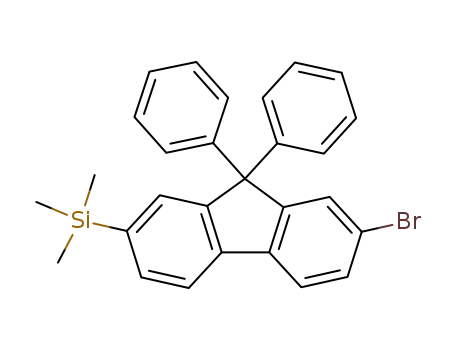
2-bromo-9,9-diphenyl-7-trimethylsilylfluorene
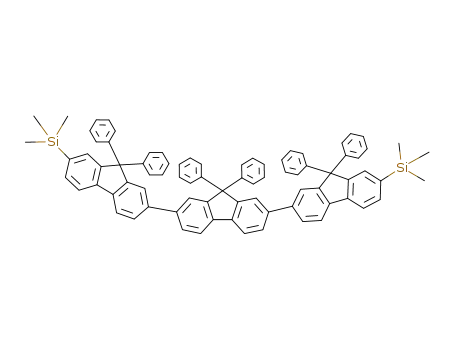
7,7'-bis(trimethylsilyl)-ter(9,9-diphenylfluorene)
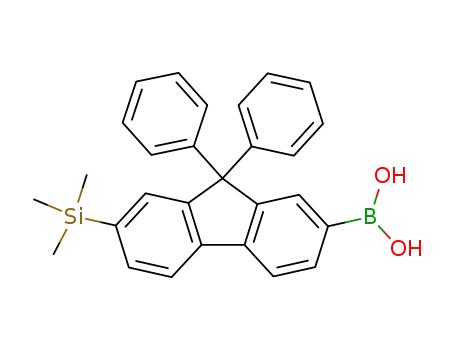
9,9-diphenyl-7-trimethylsilylfluorenyl-2-boronic acid