Your Location:Home >Products >OLED intermediates >Fluorenes >605644-46-0


Product Details
Chemical Properties
Oyster solid
-
The invention belongs to the field of organic synthesis and provides a preparation method of a novel liquid crystal material 2-(4-biphenyl)amino-9,9-dimethylfluorene. The preparation method is characterized in that fluorene is subjected to methylation with methyl iodide to obtain 9,9-dimethylfluorene, 9,9-dimethylfluorene is subjected to nitration with nitric acid to obtain 2-nitro-9,9-dimethylfluorene, 2-nitro-9,9-dimethylfluorene is subjected to hydrazine hydrate reduction to obtain 2-amino-9,9-dimethylfluorene, and 2-amino-9,9-dimethylfluorene is subjected to reaction with bromobiphenyl toobtain 2-(4-biphenyl)-amino-9,9-dimethylfluorene; the structure of the 2-(4-biphenyl)-amino-9,9-dimethylfluorene is characterized via 1H-NMR, 13C-NMR, IR and MS. The 2-(4-biphenyl)-amino-9,9-dimethylfluorene has good thermostability and high glass transition temperature and is applicable to hole transport materials in organic light-emitting devices.
This invention relates to a kind of a kind of [...] imidazole derivatives and method for preparing the same. The structure of this compound is the formula is: . This method is convenient, pervasive, economic. Type I in the organic photoelectric material and based on the analysis of the fluorescence detection has a broad application prospect in the field; in addition, because of such compounds are containing two nitrogen atom heterocyclic compound, has good biological activity, can also be used as a drug intermediate, preparing human, livestock anathematic drug. (by machine translation)
A dendrimer and an organic light-emitting device including an organic layer having the dendrimer.
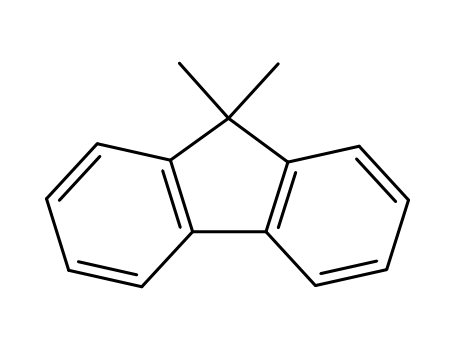
9,9-dimethyl-9H-fluorene

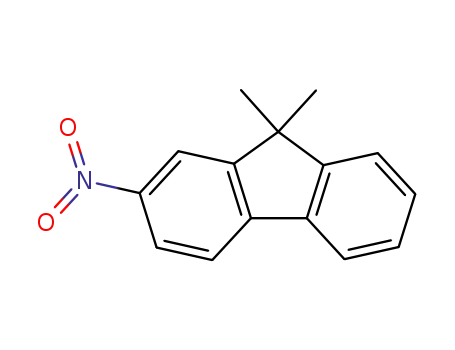
9,9-dimethyl-2-nitro-9H-indole
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; nitric acid;
|
74% |
|
With
nitric acid;
In
N,N-dimethyl-formamide;
at 70 - 85 ℃;
for 3h;
|
65% |
|
With
nitric acid; acetic acid;
|
|
|
With
nitric acid; acetic acid;
for 0.5h;
Cooling with ice;
|
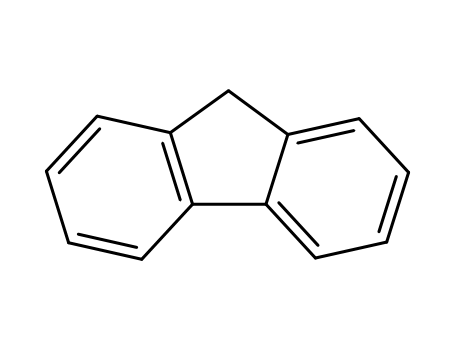
9H-fluorene


9,9-dimethyl-2-nitro-9H-indole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: potassium hydroxide / dimethyl sulfoxide / 0.5 h / 20 °C
1.2: 5 h / 20 °C
2.1: acetic acid; nitric acid / 0.5 h / Cooling with ice
With
nitric acid; acetic acid; potassium hydroxide;
In
dimethyl sulfoxide;
|

9,9-dimethyl-9H-fluorene

9H-fluorene
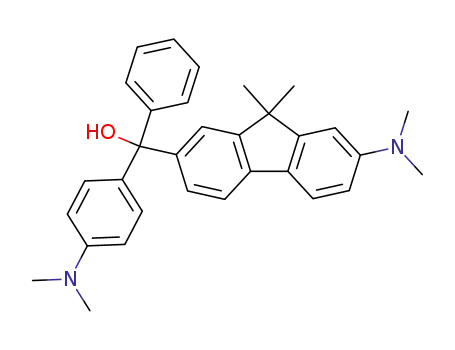
(7-Dimethylamino-9,9-dimethyl-9H-fluoren-2-yl)-(4-dimethylamino-phenyl)-phenyl-methanol
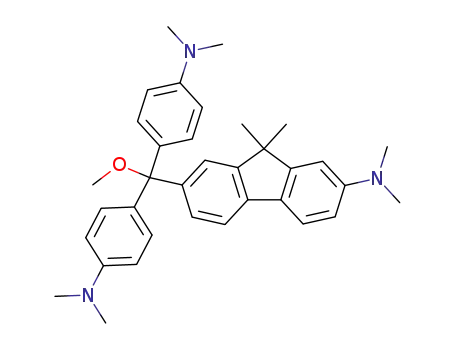
{7-[Bis-(4-dimethylamino-phenyl)-methoxy-methyl]-9,9-dimethyl-9H-fluoren-2-yl}-dimethyl-amine
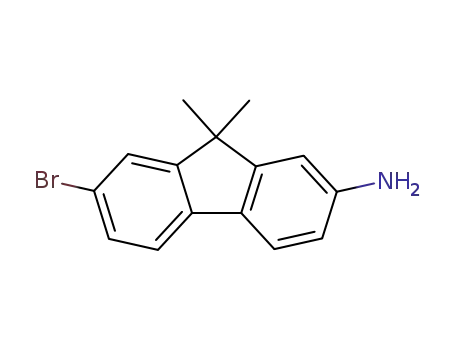
2-amino-7-bromo-9,9-dimethyl-9H-fluorene
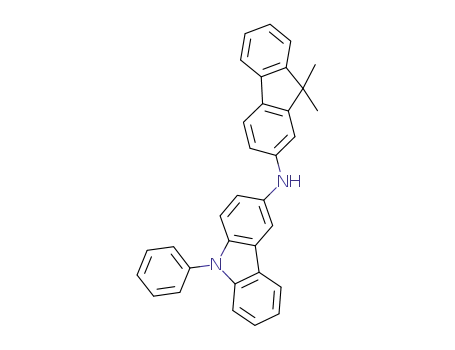
C33H26N2
CAS:7065-92-1
CAS:868549-07-9