Your Location:Home >Products >Functional intermediates >100953-52-4
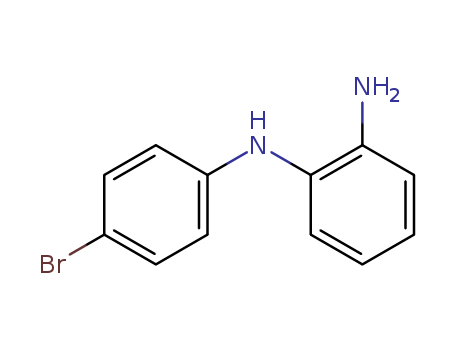

Product Details
Two triphenylamine-substituted benzimidazole derivatives were synthesized for use as efficient deep-blue emitters in nondoped fluorescent organic light-emitting diodes (FLOLEDs). The molecular design of 40,40 0-(1H-benzo[d]imidazole-1,2-diyl)bis(N,N-diphenylbiphenyl-4-amine) (T2B) to limit the molecular packing density enabled T2B-based devices to suppress the exciton quenching by a bulky three-dimensional structure. Nondoped FLOLEDs fabricated using T2B as a blue emitter exhibited an external quantum efficiency of 4.67% with color coordinates of (0.15, 0.08).
Recently, solution-processable PhOLEDs have been attracting great interest for their low cost and high productivity relative to the vacuum-deposited devices. Similar to vacuum-deposited OLEDs, however, they usually suffer from serious efficiency roll-offs, especially in high brightness. Finding a feasible way and/or designing novel materials to increase efficiencies and reduce roll-offs simultaneously are highly desired. Herein, a new family of solution-processable cyclometalated iridium(III) phosphors with carbazole (Cz) and/or diphenylphosphoryl (Ph2PO) units functionalized main ligands has been designed. Owing to Cz and Ph2PO moieties possessing bulky steric effects, they can suppress the intermolecular strong packing and then decrease TTA effects and emission quenching. Meanwhile, the resulting OLEDs based on the designed phosphors exhibit considerable efficiencies and relatively small efficiency roll-offs. The device based on 4 containing both Cz and Ph2PO units realized a maximum current efficiency of 21.3 cd A-1, accompanied by a small roll-off. By optimization of the configuration of OLEDs, the device performance can be further enhanced, demonstrating their potential for high-performance solution-processable PhOLEDs.
The invention discloses a deuterium atom-containing electron transport material and an application thereof. The deuterium atom-containing electron transport material is a benzimidazole compound with a deuterated substituent, and can be used as an electron transport material in an electroluminescent device. The novel compounds enable the organic electroluminescent device to be improved in efficiency and achieve longer device life, and better device performance can be provided. An electroluminescent device and a compound formulation are also disclosed.
Provided is a benzo imidazolone derivative organic electroluminescent compound represented by chemical formula a. Chemical Formula a. The benzo imidazolone derivative organic light emitting compound is suitable for inclusion in an organic electroluminescent device. Both electrochemical stability and thermal stability can be satisfactorily met.
A new effective approach for synthesizing diverse N-substituted-benzene-1,2-diamines is reported. The treatment of N-substituted-2-nitroanilines with thiourea dioxide in the presence of sodium hydroxide efficiently formed the corresponding N-substituted-benzene-1,2-diamines, including N-(4-chlorophenyl)benzene-1,2-diamine with a good yield of 94%. The by-product is environmentally-friendly urea and is easy to separate from the product by filtration procedure that enhances the convenience of the approach.
The invention relates to the technical field of organic electroluminescent display, particularly discloses an organic material of a polyheterocyclic compound, and also discloses application of the organic material in an organic electroluminescent device. The polyheterocyclic compound provided by the invention is shown as a general formula (I), can be applied to the field of organic electroluminescence, and is used as a luminescent layer main body material. The compound with the structure provided by the invention is applied to an OLED device, and can reduce the driving voltage and improve thelight emitting efficiency of the device.
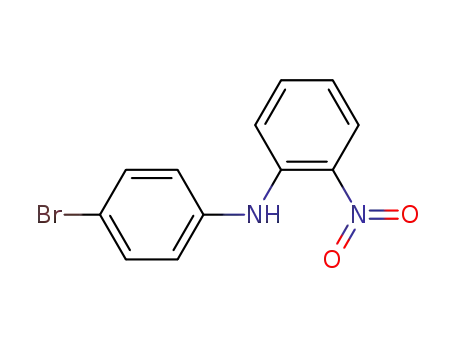
4-bromo-N-(2-nitrophenyl)benzenamine

N1-(4-bromophenyl)benzene -1,2 -diamine
| Conditions | Yield |
|---|---|
|
With
sodium hydrogensulfite;
In
tetrahydrofuran; methanol; water;
at 20 ℃;
Inert atmosphere;
|
100% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
for 5h;
Reflux;
|
100% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
for 24h;
Reflux;
|
99% |
|
With
tin(ll) chloride;
In
ethanol;
for 24h;
Reflux;
|
99% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
at 70 ℃;
for 2.83333h;
|
97% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
for 10h;
Inert atmosphere;
|
95.2% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
at 80 ℃;
for 12h;
|
94% |
|
With
Aminoiminomethanesulfinic acid; sodium hydroxide;
In
ethanol; water;
at 50 ℃;
for 1.5h;
|
91% |
|
With
tin(ll) chloride;
In
ethanol;
Reflux;
|
80% |
|
With
tin(ll) chloride;
In
ethanol;
at 75 ℃;
for 4h;
Inert atmosphere;
|
78% |
|
With
ammonium chloride; zinc;
In
tetrahydrofuran; methanol; water;
at 50 - 80 ℃;
for 12h;
|
76% |
|
With
acetic acid; zinc;
In
ethanol; water;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
75.8% |
|
With
tin(II) chloride dihdyrate;
In
ethanol;
for 3h;
Inert atmosphere;
Reflux;
Schlenk technique;
|
62% |
|
With
hydrogenchloride; ethanol; tin(ll) chloride;
|
|
|
With
methanol; sodium dithionite;
In
tetrahydrofuran; methanol; water;
at 20 ℃;
for 3h;
Product distribution / selectivity;
|
|
|
With
ethanol; tin(ll) chloride;
for 24h;
Reflux;
|
|
|
With
hydrogenchloride; indium;
In
water;
at 100 ℃;
for 1h;
|
|
|
With
ethanol; tin(ll) chloride;
for 24h;
Reflux;
|
|
|
With
tin(ll) chloride;
In
ethanol;
|
|
|
With
hydrogenchloride; iron;
In
water;
at 90 ℃;
|
|
|
With
iron; acetic acid;
In
water; ethyl acetate;
for 6h;
Reflux;
|
|
|
With
iron; ammonium chloride;
In
ethanol; water;
at 90 ℃;
for 3h;
|
|
|
With
hydrogenchloride; iron;
In
methanol; water;
Heating;
|
|
|
With
ethanol; tin(ll) chloride;
for 3h;
Inert atmosphere;
Reflux;
|
|
|
With
acetic acid; zinc;
In
dichloromethane;
at 20 ℃;
|
|
|
With
sodium sulfide; water;
In
ethanol;
for 3h;
Inert atmosphere;
|
|
|
With
iron; acetic acid;
In
water; ethyl acetate;
for 6h;
Reflux;
|
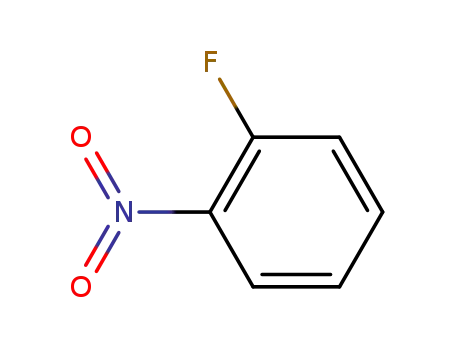
ortho-nitrofluorobenzene

N1-(4-bromophenyl)benzene -1,2 -diamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: water / 2.5 h / 100 °C
2: indium; hydrogenchloride / water / 1 h / 100 °C
With
hydrogenchloride; indium;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 72 h / 170 - 180 °C
2: tin(II) chloride dihdyrate / ethanol / 24 h / Reflux
With
potassium fluoride; tin(II) chloride dihdyrate;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: dimethyl sulfoxide / 48 h / 100 °C / Inert atmosphere; Schlenk technique
2: tin(II) chloride dihdyrate / ethanol / 3 h / Inert atmosphere; Reflux; Schlenk technique
With
tin(II) chloride dihdyrate;
In
ethanol; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride dihydrate / 30 h / 170 - 180 °C / Inert atmosphere
2: tin(II) chloride dihdyrate / ethanol / 10 h / Inert atmosphere
With
potassium fluoride dihydrate; tin(II) chloride dihdyrate;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 72 h / 170 °C
2: tin(ll) chloride / ethanol / 24 h / Reflux
With
potassium fluoride; tin(ll) chloride;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 72 h / 180 °C
2: tin(II) chloride dihdyrate / ethanol / 12 h / 80 °C
With
potassium fluoride; tin(II) chloride dihdyrate;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 °C
1.2: 16 h / 25 °C
2.1: acetic acid; iron / water; ethyl acetate / 6 h / Reflux
With
iron; sodium hydride; acetic acid;
In
water; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: triethylamine / 4 h / 150 °C / Microwave irradiaton
1.2: 0.25 h
2.1: tin(II) chloride dihdyrate / ethanol / 2.83 h / 70 °C
With
tin(II) chloride dihdyrate; triethylamine;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 18 h / 150 °C / Inert atmosphere
2: zinc; ammonium chloride / tetrahydrofuran; water; methanol / 12 h / 50 - 80 °C
With
potassium fluoride; ammonium chloride; zinc;
In
tetrahydrofuran; methanol; water;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 30 h / 170 - 180 °C
2: tin(II) chloride dihdyrate / ethanol / 5 h / Reflux
With
potassium fluoride; tin(II) chloride dihdyrate;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: dimethyl sulfoxide / 48 h / 100 °C / Inert atmosphere
2: tin(ll) chloride; ethanol / 3 h / Inert atmosphere; Reflux
With
ethanol; tin(ll) chloride;
In
dimethyl sulfoxide;
|
|
|
Multi-step reaction with 2 steps
1: potassium fluoride / 160 °C
2: acetic acid; zinc / dichloromethane / 20 °C
With
potassium fluoride; acetic acid; zinc;
In
dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: 30 h / 180 °C / Inert atmosphere
2: sodium sulfide; water / ethanol / 3 h / Inert atmosphere
With
sodium sulfide; water;
In
ethanol;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydride / N,N-dimethyl-formamide / 16 h / 20 °C
2: iron; acetic acid / water; ethyl acetate / 6 h / Reflux
With
iron; sodium hydride; acetic acid;
In
water; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: dimethyl sulfoxide / 18 h / Inert atmosphere; Reflux
2: zinc; acetic acid / ethanol; water / 2 h / 0 - 20 °C / Inert atmosphere
With
acetic acid; zinc;
In
ethanol; water; dimethyl sulfoxide;
|
|
|
Multi-step reaction with 2 steps
1: dimethyl sulfoxide / 48 h / 100 °C / Inert atmosphere
2: tin(ll) chloride / ethanol / 4 h / 75 °C / Inert atmosphere
With
tin(ll) chloride;
In
ethanol; dimethyl sulfoxide;
|

4-bromo-N-(2-nitrophenyl)benzenamine
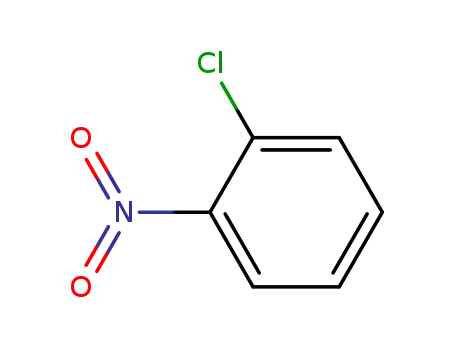
2-Chloronitrobenzene
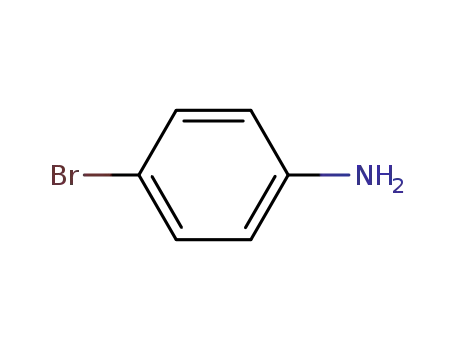
4-bromo-aniline

ortho-nitrofluorobenzene
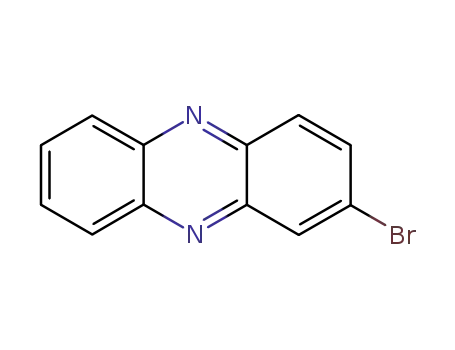
2-bromophenazine
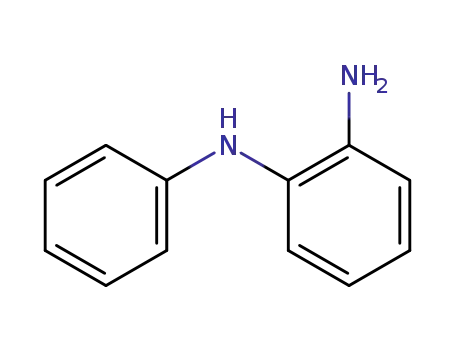
N-phenyl-1,2-benzenediamine
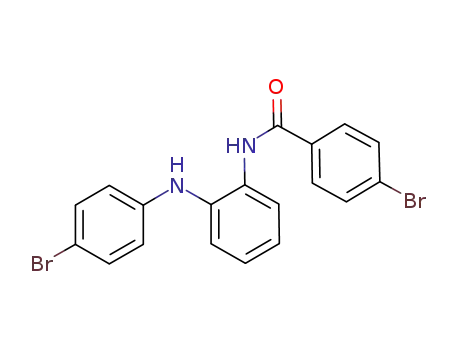
C19H14Br2N2O
1,2-bis(4-bromophenyl)-1H-benzo[d]imidazole
CAS:3460-18-2
CAS:128055-74-3
Molecular Formula:C25H12Br4
Molecular Weight:632
CAS:1160294-93-8
CAS:1228468-73-2