Your Location:Home >Products >OLED intermediates >Carbazoles >1428551-28-3

Product Details
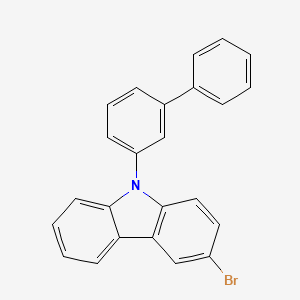
| Description | 9-([1,1'-Biphenyl]-3-yl)-3-bromo-9H-carbazole is an organic compound consists of a carbazole unit substituted with a 3-brominated biphenyl moiety. 9-([1,1'-Biphenyl]-3-yl)-3-bromo-9H-carbazole is a solid at room temperature and exhibits interesting photophysical and electronic properties, making it potentially useful in the field of organic electronics. The presence of the bromine substituent and the biphenyl group provides opportunities for further chemical functionalization and integration into various optoelectronic devices, such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and other advanced materials. The unique structural features of 9-([1,1'-biphenyl]-3-yl)-3-bromo-9H-carbazole contribute to its potential applications in areas like organic semiconductors, photovoltaics, and luminescent materials. |
|
Application |
9-([1,1'-Biphenyl]-3-yl)-3-bromo-9H-carbazole (cas# 1428551-28-3) is used as the OLED. |
| XLogP3-AA | 7.3 |
| Complexity | 473 |
Isomeric SMILES: C1=CC=C(C=C1)C2=CC(=CC=C2)N3C4=C(C=C(C=C4)Br)C5=CC=CC=C53
InChIKey: NSRPRPVECXNOLB-UHFFFAOYSA-N
InChI: InChI=1S/C24H16BrN/c25-19-13-14-24-22(16-19)21-11-4-5-12-23(21)26(24)20-10-6-9-18(15-20)17-7-2-1-3-8-17/h1-16H
The invention relates to an organic elec...
An object of the present invention is to...
The invention relates to a method for sy...
9-([1,1'-biphenyl]-3-yl)-9H-carbazole

9-([1,1′-biphenyl]-3-yl)-3-bromo-9H-carbazole
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 12h; Reflux;
|
90% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 12h;
|
88% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 12h; Cooling with ice; Darkness;
|
88% |
|
With N-Bromosuccinimide; In chloroform; for 5h; Reflux;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 12h;
|
40 g |
|
With N-Bromosuccinimide; at 20 ℃;
|
3-bromo-9H-carbazole

3-iodo-1,1'-biphenyl

9-([1,1′-biphenyl]-3-yl)-3-bromo-9H-carbazole
| Conditions | Yield |
|---|---|
|
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100 ℃;
|
84% |
|
With tri-tert-butyl phosphine; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate; In toluene; at 100 ℃; for 10h; Inert atmosphere;
|
51% |
3-bromo-9H-carbazole
3-iodo-1,1'-biphenyl
9-([1,1'-biphenyl]-3-yl)-9H-carbazole
9-(3-bromophenyl)carbazole
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97