Your Location:Home >Products >OLED intermediates >Carbazoles >185112-61-2

Product Details
|
Chemical Properties |
White solid |
| XLogP3-AA | 5.7 |
| Complexity | 322 |
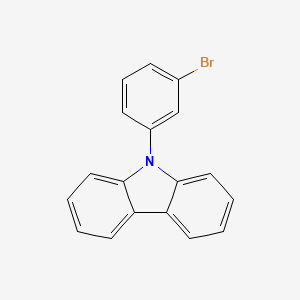
N-(3-Bromophenyl)carbazole is an aromatic organic compound consists of a carbazole moiety substituted with a bromine atom at the 3-position of the phenyl ring. This compound is a solid at room temperature and exhibits interesting photophysical and electrochemical properties. N-(3-Bromophenyl)carbazole has potential applications in the field of organic electronics, such as in the development of organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and other optoelectronic devices, due to its unique electronic and charge transport characteristics. 9-(3-Bromophenyl)carbazole can be used in dyes, pigments, and intermediates of synthetic materials.
Isomeric SMILES: C1=CC=C2C(=C1)C3=CC=CC=C3N2C4=CC(=CC=C4)Br
InChIKey: ZKGHGKNHPPZALY-UHFFFAOYSA-N
InChI: InChI=1S/C18H12BrN/c19-13-6-5-7-14(12-13)20-17-10-3-1-8-15(17)16-9-2-4-11-18(16)20/h1-12H
Two isomeric host materials were synthes...
A new bipolar host material (CzPhPz) con...
Inexpensive and eco-friendly luminescent...
We have synthesized a series of novel hy...
An organic compound, an organic light em...
The invention relates to a method for sy...
1-Bromo-3-iodobenzene

9H-carbazole

9-(3-bromophenyl)carbazole
| Conditions | Yield |
|---|---|
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃;
|
98% |
|
With potassium phosphate; copper(l) iodide; trans-1,2-cyclohexanediamine; In toluene; for 4h;
|
96% |
|
With palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate; In toluene; at 110 ℃;
|
90% |
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; for 24h; Inert atmosphere;
|
89% |
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; for 24h;
|
89% |
|
With copper(I) oxide; 2,2,6,6-tetramethylheptane-3,5-dione; potassium hydroxide; In N,N-dimethyl-formamide; at 110 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
84% |
|
With copper; potassium carbonate; at 150 ℃; for 48h; Inert atmosphere;
|
82.4% |
|
With 18-crown-6 ether; copper; potassium carbonate; In N,N-dimethyl-formamide; at 150 ℃; Inert atmosphere;
|
79% |
|
With copper; potassium carbonate; In Tetraethylene glycol dimethyl ether; at 140 ℃; for 5h;
|
78% |
|
With copper(l) iodide; potassium carbonate; In 5,5-dimethyl-1,3-cyclohexadiene; for 48h; Inert atmosphere; Reflux;
|
78% |
|
With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; tri tert-butylphosphoniumtetrafluoroborate; In toluene; for 3h; Inert atmosphere; Reflux;
|
77% |
|
With dibenzo-18-crown-6; copper; potassium carbonate; In N,N-dimethyl-formamide; for 7h; Inert atmosphere; Reflux;
|
71% |
|
With potassium phosphate; copper(l) iodide; In 1,4-dioxane; at 90 ℃; for 12h; Inert atmosphere;
|
70% |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate; In toluene; at 80 ℃;
|
70% |
|
With potassium phosphate; copper(II) iodide; rac-diaminocyclohexane; In 1,4-dioxane; for 16h; Inert atmosphere; Reflux;
|
69% |
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 150 ℃; for 48h; Inert atmosphere;
|
65% |
|
With 18-crown-6 ether; copper; potassium carbonate; In N,N-dimethyl-formamide; for 24h; Sealed tube; Inert atmosphere; Reflux;
|
64.8% |
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; for 24h; Inert atmosphere;
|
58% |
|
With potassium carbonate; rac-diaminocyclohexane; copper(l) chloride; In dimethyl sulfoxide; at 110 ℃; for 16h; Inert atmosphere;
|
46% |
|
With copper(l) iodide; 18-crown-6 ether; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; for 24h;
|
36% |
|
With tri-tert-butyl phosphine; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate; In toluene; at 100 ℃; for 16h; Inert atmosphere; Reflux;
|
35% |
|
With sodium t-butanolate; 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); In xylene; for 24h; Inert atmosphere; Reflux;
|
|
|
With copper; potassium carbonate; In N,N-dimethyl-formamide;
|
|
|
With Cu-catalyst; In dichlorobenzene;
|
|
|
With dibenzo-18-crown-6; copper; potassium carbonate; In 1,2-dichloro-benzene; at 100 ℃; for 12h; Inert atmosphere;
|
|
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃;
|
|
|
With potassium carbonate; In 1,2-dichloro-benzene;
|
|
|
With copper(l) iodide; Reflux;
|
|
|
1-Bromo-3-iodobenzene; With tri-tert-butyl phosphine; palladium diacetate; In toluene; at 20 ℃; for 1h; Inert atmosphere;
9H-carbazole; In toluene; at 80 ℃; for 6h; Inert atmosphere;
|
1.9 g |
|
With copper(l) iodide; (-)-(1R,2R)-diaminocyclohexane; Reflux;
|
|
|
With dibenzo-18-crown-6; copper; potassium carbonate; In 1,2-dichloro-benzene; at 100 ℃;
|
|
|
With potassium phosphate; copper(l) iodide; In 1,4-dioxane; at 90 ℃; for 12h;
|
|
|
With potassium phosphate; copper(l) iodide; In 1,4-dioxane; at 90 ℃; for 12h;
|
|
|
With copper(l) iodide; potassium carbonate; In N,N-dimethyl-formamide; at 150 ℃; for 12h;
|
1,3-dibromobenzene

9H-carbazole

9-(3-bromophenyl)carbazole
| Conditions | Yield |
|---|---|
|
With potassium phosphate; copper(l) iodide; ethylenediamine; In toluene; at 120 ℃; for 24h;
|
85% |
|
With copper; potassium carbonate; nitrobenzene; at 120 - 160 ℃; Inert atmosphere;
|
85% |
|
With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine; In 5,5-dimethyl-1,3-cyclohexadiene; at 120 ℃; for 24h; Inert atmosphere;
|
83% |
|
With copper; potassium carbonate; In N,N-dimethyl-formamide; at 130 ℃; Inert atmosphere;
|
71% |
|
|
47% |
|
With copper(l) iodide; potassium carbonate; In N,N-dimethyl-formamide;
|
|
|
With copper(l) iodide; calcium carbonate; at 158 ℃; Inert atmosphere;
|
|
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; potassium tert-butylate; In 5,5-dimethyl-1,3-cyclohexadiene; for 6h; Reflux;
|
80 mmol |
N-pentanoyl-N-[[2'-(1H-tetrazole-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine methyl-ester
1-Bromo-3-iodobenzene
9H-carbazole
cis,trans-2,5-dimethoxytetrahydrofuran
9-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborane-2-yl)phenyl)-9-hydrogencarbazole
2,9-bis(3-(9H-carbazol-9-yl)phenyl)-4,7-diphenyl-1,10-phenanthroline
3,3''-di(9H-carbazol-9-yl)-1,1':3',1''-terphenyl
2,6-bis(3-(9H-carbazol-9-yl)phenyl)-pyridine
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97
CAS:1150-62-5