Your Location:Home >Products >OLED intermediates >Fluorenes >86-76-0

Product Details
|
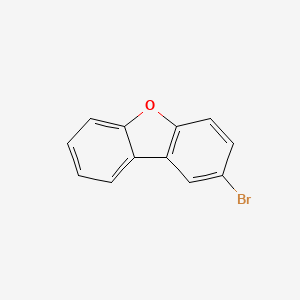
Description |
2-Bromo-dibenzofuran is the bromo modified form of dibenzofuran, an aromatic compound consisting two benzene rings fused to a central furan ring. It can be used as a reagent in Friedel-Crafts acylation reactions. |
|
Chemical Properties |
off-white powder |
|
Uses |
2-Bromodibenzofuran is used as a reagent in Friedel-Crafts acylation reactions. Used as an intermediate in medicine, organic synthesis and materials. |
| Supplier | Puyang Huicheng Electronic Material Co., Ltd was founded in 2002, which is located in Puyang Economic and Technological Development Zone. It is a national high-tech and listed enterprise which is specialized in research, production, trade of acid anhydrides and other functional material intermediates. The stock code is 300481. Our company has independent import and export rights and our products have been exported to Europe, America and Southeast Asia. |
4DBFCz was synthesized according to the synthetic method used for 2DBFCz with 4-bromodibenzo[b,d]furan (1.0 g, 4.0 mmol), instead of 2-bromodibenzo[b,d]furan. 4DBFCz was …
SiDBFCz was synthesized by first Ullmann type CN coupling reaction of 2-bromodibenzo[b,d]furan and a carbazole group, forming the intermediate compound DBFCz. The subsequent …
An efficient transformation of dibenzoxa...
dibenzofuran

2,8-dibromodibenzofuran

2-bromodibenzo[b,d]furan
| Conditions | Yield |
|---|---|
|
With bromine; In chloroform; at 20 ℃; for 240h;
|
47% |

2,8-dibromodibenzofuran

2-bromodibenzo[b,d]furan
| Conditions | Yield |
|---|---|
|
|
50% |
dibenzofuran
dibenzo[b,d]furan-2-amine
5-bromo-2-phenoxyaniline
2-(4-bromo-phenoxy)-aniline
2-bromo-8-(phenylacetyl)dibenzofuran
2,8-dibromodibenzofuran
dibenzofuran
2-bromo-dibenzofuran-4-carboxylic acid
CAS:97511-05-2