Your Location:Home >Products >OLED intermediates >Fluorenes >171408-76-7

Product Details
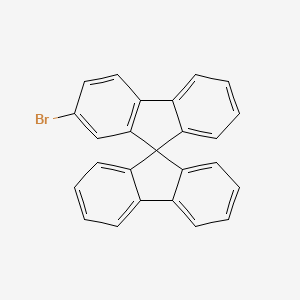
Puyang Huicheng Electronic Materials Co., Ltd., established in 2002, is a national high-tech enterprise renowned for its expertise in functional material intermediates. Located in the Puyang Economic and Technological Development Zone, our company excels in innovation, quality, and customer satisfaction. One of our premier products, 2-Bromo-9,9'-spirobifluorene, embodies our commitment to advancing technological applications and meeting the diverse needs of our global clients.
The Essential Value of 2-Bromo-9,9'-Spirobifluorene
2-Bromo-9,9'-spirobifluorene is a versatile chemical compound with significant applications in both the pharmaceutical and optoelectronic industries. This off-white to light yellow crystal or powder is a derivative of 9,9'-spirobifluorene, offering unique properties that make it indispensable for advanced material synthesis:
Pharmaceutical Intermediates: Acts as a crucial intermediate in the synthesis of various pharmaceutical compounds, facilitating drug development and research.
Optoelectronic Materials: Essential in the creation of blue light-emitting materials for organic light-emitting diodes (OLEDs), enhancing display technologies with high photoluminescence efficiency and excellent chemical stability.
Superior Performance for Advanced Applications
2-Bromo-9,9'-spirobifluorene stands out due to its remarkable characteristics:
High Photoluminescence Efficiency: Ensures bright and efficient blue light emission, vital for high-performance OLEDs.
Chemical Stability: Provides durability and reliability in various applications, from pharmaceuticals to optoelectronics.
Thermal Stability: Exhibits a high glass-transition temperature, maintaining stability under high-temperature conditions.
Color Tenability: Allows for easy color adjustment through the introduction of low band gap co-monomers, enabling versatile design and application possibilities.
N-Substitution in perylene diimide (PDI)...
A high efficient UV-violet emission type...
As we know, 2-bromo-9,9′-spirobifluorene can be readily synthesized from 2-bromo-9-… (7) However, this method is unsuitable for the synthesis of 4-bromo-9,9′-spirobifluorene …
2-bromofluoren-9-one
2-iodobiphenyl
9-(biphenyl-2-yl)-2-bromo-9-fluorenol
2-biphenylmagnesium bromide
2′-cyano-9,9-spirobifluorene
9,9'-spirobi[9H-fluoren]-2'-yl-boronic acid
2-[N-(9-phenylcarbazole-3-yl)-N-phenylamino]-spiro-9,9'-bifluorene
C68H41N
CAS:28320-31-2
Molecular Formula:C<sub>15</sub>H<sub>13</sub>Br
Molecular Weight:273.17
CAS:171408-84-7
Molecular Formula:C25H14Br2
Molecular Weight:474.2