Your Location:Home >Products >Functional intermediates >1160294-93-8
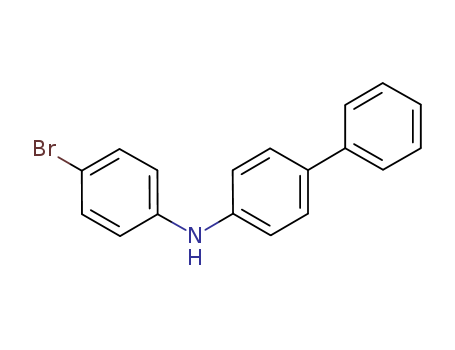

Product Details
Uses
N-(4-Bromophenyl)-[1,1-biphenyl]-4-amine is a useful reagent for preparing carbazole and dibenzofuran-linked tertiary arylamine compounds, which may improve electroluminescent properties.
The present invention is related to a first compound for an organic optoelectronic device represented by Chemical Formula 1, a composition for an organic optoelectronic device including the same, an organic optoelectronic device, and a display device. In Chemical Formula 1, definitions of each substituent are the same as defined in the specification.
An organic optoelectronic device and a display device including the organic optoelectronic device, the organic optoelectronic device including an anode and a cathode facing each other, a light emitting layer between the anode and the cathode, a hole transport layer between the anode and the light emitting layer, and a hole transport auxiliary layer between the light emitting layer and the hole transport layer, wherein the light emitting layer includes a first compound represented by Chemical Formula 1 and a second compound represented by Chemical Formula 2, and the hole transport auxiliary layer includes a third compound represented by Chemical Formula 3,
An organic optoelectric deviceincludes an anode and a cathode facing each other, an emission layer between the anode and the cathode, a hole transport layer between the anode and the emission layer, and a hole transport auxiliary layer between the hole transport layer and the emission layer, wherein the hole transport auxiliary layer includes a first compound represented by Chemical Formula 1 and a second compound represented by Chemical Formula 2 or a combination of Chemical Formulae 3 and 4, and a display device is disclosed. Explanations of Chemical Formulae 1 to 3 are the same as described in specification.
The present invention relates to an organic light emitting compound used in an electroluminescent device. The electroluminescent compound is represented by the chemical formula 1, and has one or more substituents represented by the structural formula 1 or structural formula 2. The electroluminescent device may be realized with excellent light emitting properties such as a driving voltage, brightness, long lifespan, etc. when the electroluminescent compound is used as a phosphorescent host compound in a hole transporting function layer or a light emitting layer.COPYRIGHT KIPO 2016
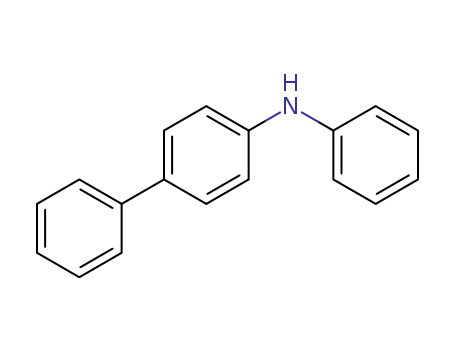
4-phenyldiphenylamine

![N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine](/upload/2023/2/5f619a77-554f-456b-9a90-7a38f5c5c725.png)
N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 25.5h;
Inert atmosphere;
Cooling with ice;
|
95.4% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 0 ℃;
for 8h;
|
95% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 0 ℃;
for 8h;
Reflux;
|
95% |
|
With
N-Bromosuccinimide;
In
ethyl acetate;
at 20 ℃;
for 24h;
|
73% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
Inert atmosphere;
|
67% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 4h;
Inert atmosphere;
|
62% |

![N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine](/upload/2023/2/5f619a77-554f-456b-9a90-7a38f5c5c725.png)
N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine
| Conditions | Yield |
|---|---|
|
With
bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate;
In
toluene;
at 95 ℃;
for 4h;
|
84% |
|
With
bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate;
In
toluene;
at 95 ℃;
for 4h;
|
81% |

4-phenyldiphenylamine
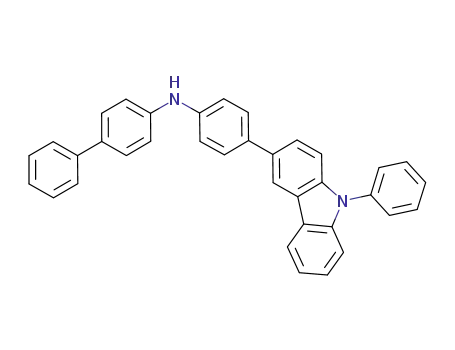
N‐[4‐(9‐phenyl‐9H‐carbazol‐3‐yl)phenyl]‐[1,1'‐biphenyl]‐4‐amine
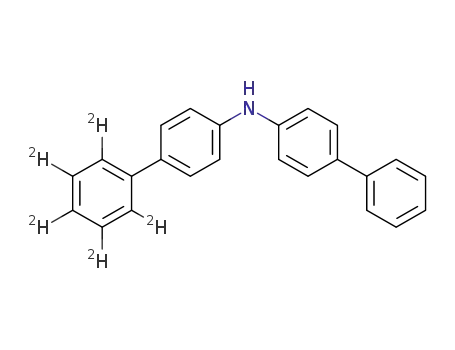
(biphenyl-4-yl)-[(biphenyl-1',2',3',5',6'-d5)-4-yl]amine
4,4'-bis{(biphenyl-4-yl)-[(biphenyl-1',2',3',5',6'-d5)-4-yl]amino}biphenyl

N-([1,1’-biphenyl]-4-yl)-[1,1:3,1“-terphenyl]-4-amine
CAS:14647-23-5
Molecular Formula:C26H24Cl2NiP2
Molecular Weight:528
CAS:152670-41-2
CAS:100953-52-4